Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question #6 For 0.4847 M KNO, what is the pH of a 3.367e-2 solution of acetic acid? Again, using the two alternate definitions of
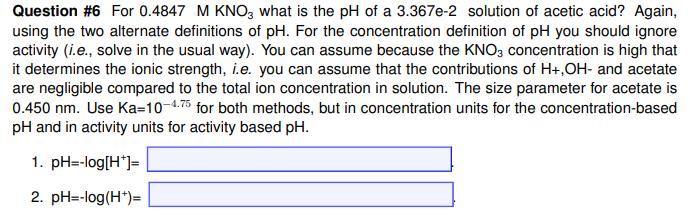
Question #6 For 0.4847 M KNO, what is the pH of a 3.367e-2 solution of acetic acid? Again, using the two alternate definitions of pH. For the concentration definition of pH you should ignore activity (i.e., solve in the usual way). You can assume because the KNO3 concentration is high that it determines the ionic strength, i.e. you can assume that the contributions of H+,OH- and acetate are negligible compared to the total ion concentration in solution. The size parameter for acetate is 0.450 nm. Use Ka=10-4.75 for both methods, but in concentration units for the concentration-based pH and in activity units for activity based pH. 1. pH=-log[H*]= 2. pH=-log(H*)=
Step by Step Solution
★★★★★
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Let us denote the ions as H and A So Firstly the co...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


