Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question 6 please 4. For the following proton transfer reactions: Label the acid (A), base (B), conjugate acid (CA) and conjugate base (CB) Add the
question 6 please 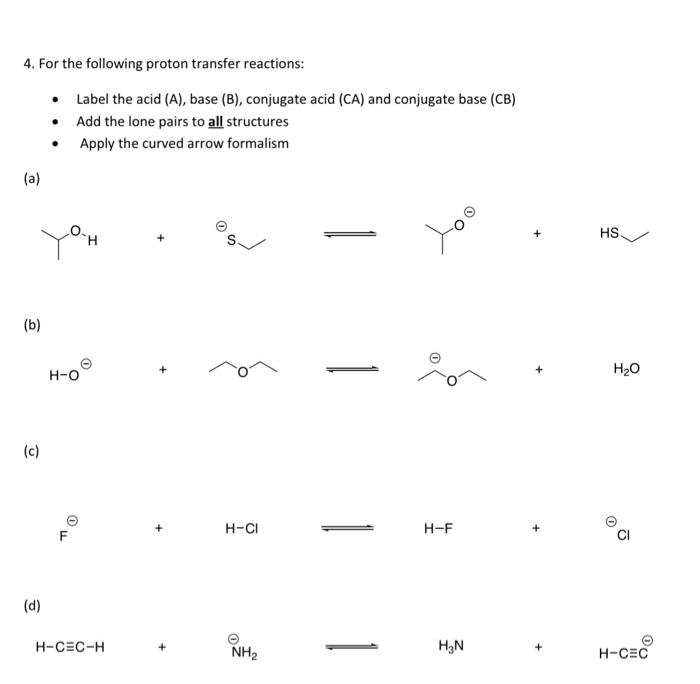

4. For the following proton transfer reactions: Label the acid (A), base (B), conjugate acid (CA) and conjugate base (CB) Add the lone pairs to all structures Apply the curved arrow formalism . (a) O )o HS (b) 0 0 H-O H2O (c) 0 o H-CI H-F CI (d) H-CEC-H NH2 HEN + H-CEC 6. Calculate the equilibrium constant, Kes, for the proton transfer reactions in Problem 4. Show your work (a) K- (b) Ke (c) Keq = (d) Ke 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


