Answered step by step
Verified Expert Solution
Question
1 Approved Answer
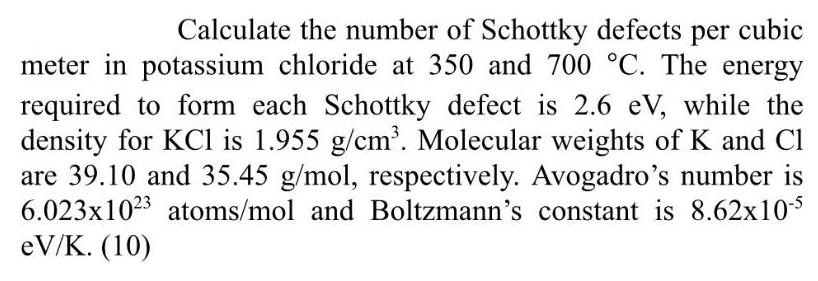
Calculate the number of Schottky defects per cubic meter in potassium chloride at 350 and 700 C. The energy required to form each Schottky
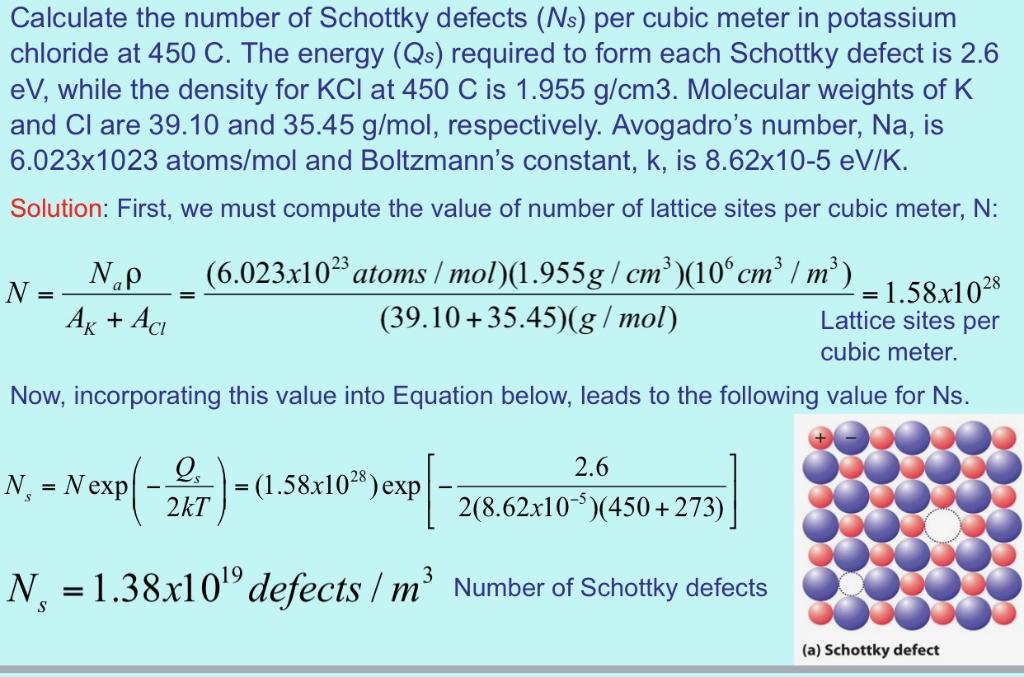
Calculate the number of Schottky defects per cubic meter in potassium chloride at 350 and 700 C. The energy required to form each Schottky defect is 2.6 eV, while the density for KCl is 1.955 g/cm. Molecular weights of K and Cl are 39.10 and 35.45 g/mol, respectively. Avogadro's number is 6.023x1023 atoms/mol and Boltzmann's constant is 8.62x105 eV/K. (10) Calculate the number of Schottky defects (Ns) per cubic meter in potassium chloride at 450 C. The energy (Qs) required to form each Schottky defect is 2.6 eV, while the density for KCI at 450 C is 1.955 g/cm3. Molecular weights of K and Cl are 39.10 and 35.45 g/mol, respectively. Avogadro's number, Na, is 6.023x1023 atoms/mol and Boltzmann's constant, k, is 8.62x10-5 eV/K. Solution: First, we must compute the value of number of lattice sites per cubic meter, N: (6.023x102atoms / mol)(1.955g/ cm )(10cm / m (39.10 +35.45)(g / mol) N.p = 1.58x1028 Lattice sites per N Ag + Ac cubic meter. Now, incorporating this value into Equation below, leads to the following value for Ns. N, = Nexp[-2xT) 2.6 = (1.58x102*) exp 2(8.62.x10)(450+ 273) N. = 1.38x10" defects / m' Number of Schottky defects %3D (a) Schottky defect
Step by Step Solution
★★★★★
3.44 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
For T 350 o C N NApA K A cl 6031023 1955 10639103545 158 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6361ebb760fde_234124.pdf
180 KBs PDF File
6361ebb760fde_234124.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


