Answered step by step
Verified Expert Solution
Question
1 Approved Answer
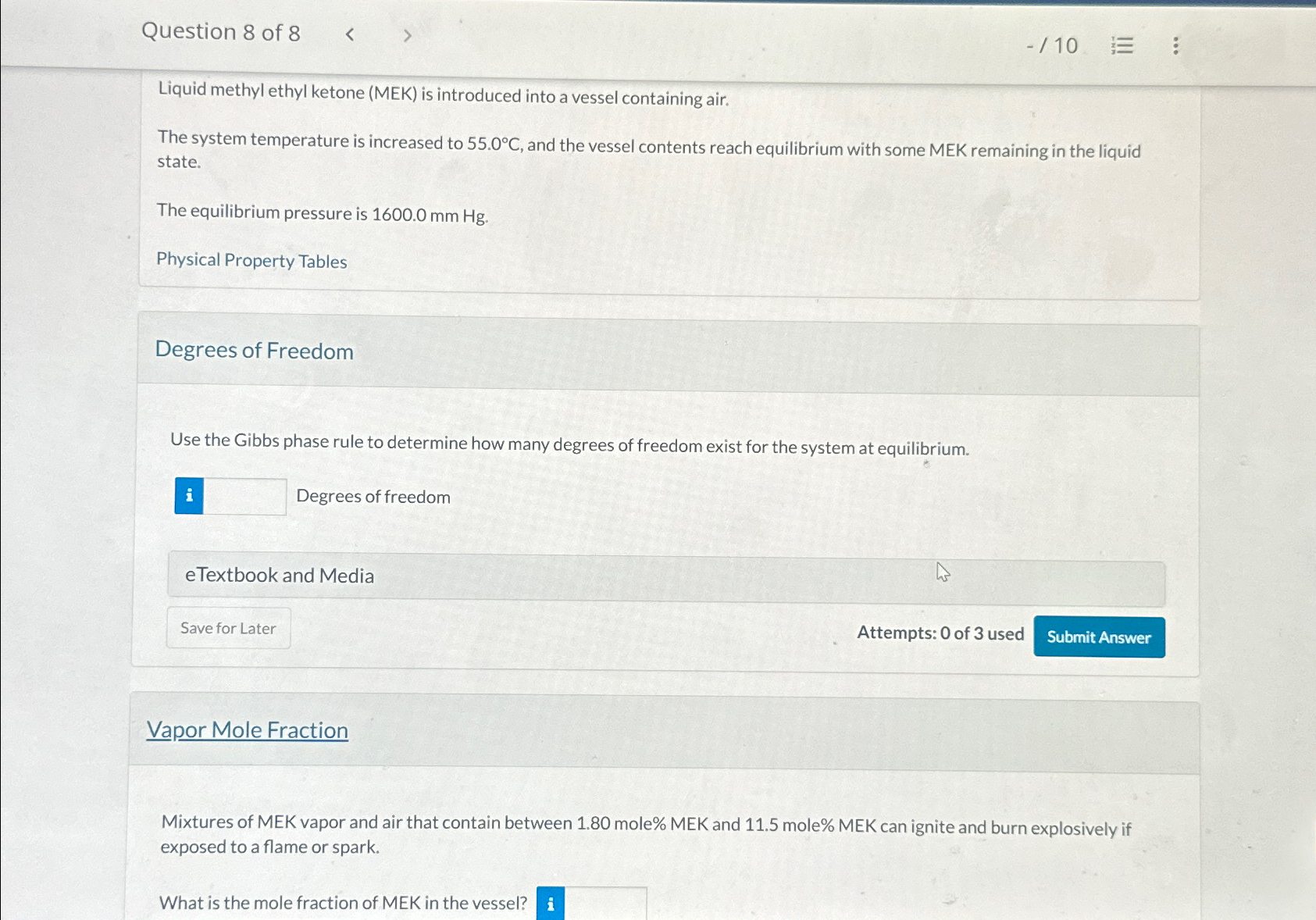
Question 8 of 8 - 1 0 Liquid methyl ethyl ketone ( MEK ) is introduced into a vessel containing air. The system temperature is
Question of
Liquid methyl ethyl ketone MEK is introduced into a vessel containing air.
The system temperature is increased to and the vessel contents reach equilibrium with some MEK remaining in the liquid state.
The equilibrium pressure is
Physical Property Tables
Degrees of Freedom
Use the Gibbs phase rule to determine how many degrees of freedom exist for the system at equilibrium.
i
Degrees of freedom
eTextbook and Media
Attempts: of used
Vapor Mole Fraction
Mixtures of MEK vapor and air that contain between mole MEK and mole MEK can ignite and burn explosively if exposed to a flame or spark.
What is the mole fraction of MEK in the vessel?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


