Question
Consider the reaction: 2,0(g) o, (9) +2Na(9). Which of the following will cause a shift in the equilibrium to the right? 1. Add more
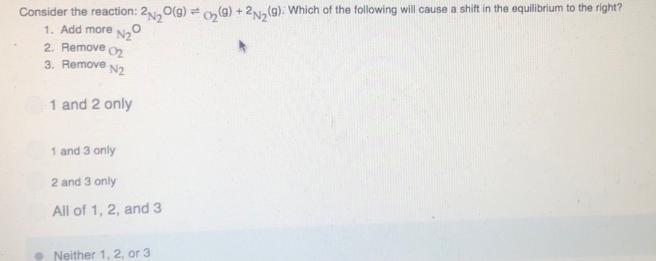
Consider the reaction: 2,0(g) o, (9) +2Na(9). Which of the following will cause a shift in the equilibrium to the right? 1. Add more Na 2. Remove o2 3. Remove N2 1 and 2 only 1 and 3 only 2 and 3 only All of 1, 2, and 3 Neither 1, 2, or 3
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Answer Option 3 1st and 3rd reaction Only the euations 1 and 3 are oxidation reduction reactions whi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Exploring Economics
Authors: Robert L Sexton
5th Edition
978-1439040249, 1439040249
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



