Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question B2 please Question B1 Question B2 Question B3 Measured Galvanic Cell Ecell (V) Cathode Material (metal) Anode Material (metal) Cathode Anode Half-Reaction Half-Reaction Reduction
question B2 please 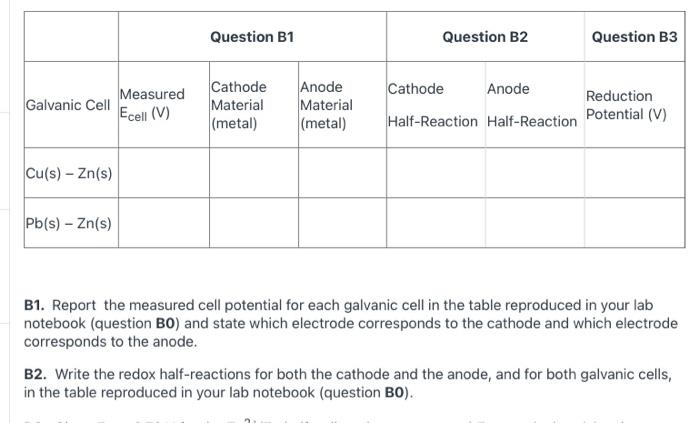
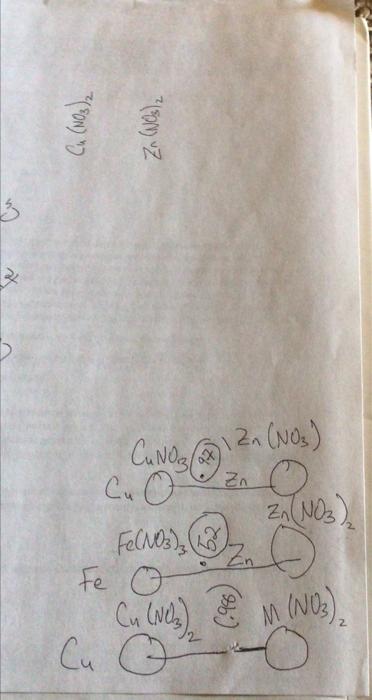
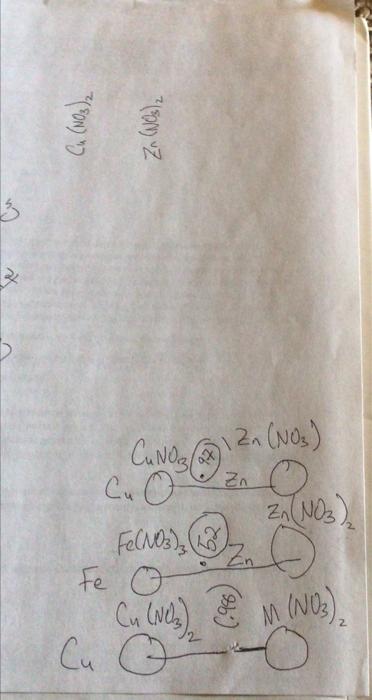
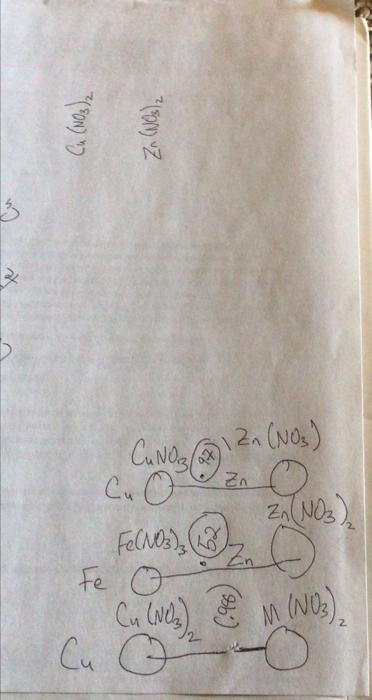
Question B1 Question B2 Question B3 Measured Galvanic Cell Ecell (V) Cathode Material (metal) Anode Material (metal) Cathode Anode Half-Reaction Half-Reaction Reduction Potential (V) Cu(s) - Zn(s) Pb(s) - Zn(s) B1. Report the measured cell potential for each galvanic cell in the table reproduced in your lab notebook (question BO) and state which electrode corresponds to the cathode and which electrode corresponds to the anode. B2. Write the redox half-reactions for both the cathode and the anode, and for both galvanic cells, in the table reproduced in your lab notebook (question BO). Cu(NO3)2 Zn (NO3)2 3 x CANO ax Zn Zn(NO3) Cu o Zn(NO3), Fe(NO3) , 2 o Fe o Cu (Nez & M (NO) Cu o ) Cu(NO3)2 Zn (NO3)2 3 x CANO ax Zn Zn(NO3) Cu o Zn(NO3), Fe(NO3) , 2 o Fe o Cu (Nez & M (NO) Cu o ) Cu(NO3)2 Zn (NO3)2 3 x CANO ax Zn Zn(NO3) Cu o Zn(NO3), Fe(NO3) , 2 o Fe o Cu (Nez & M (NO) Cu o ) 
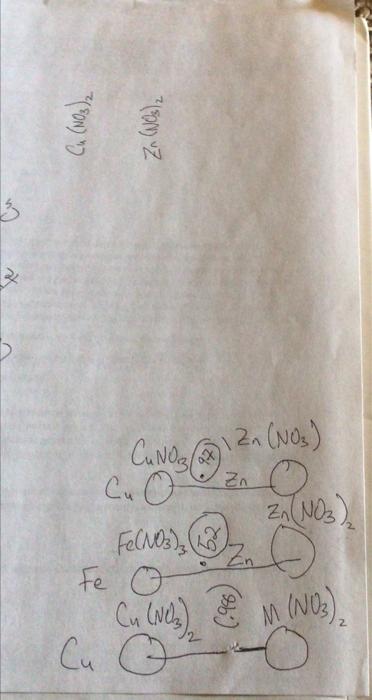
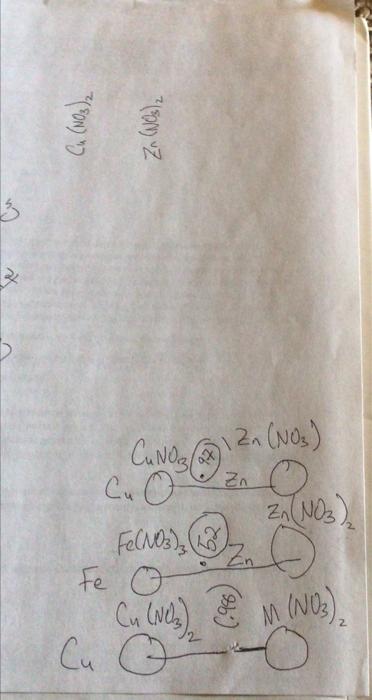
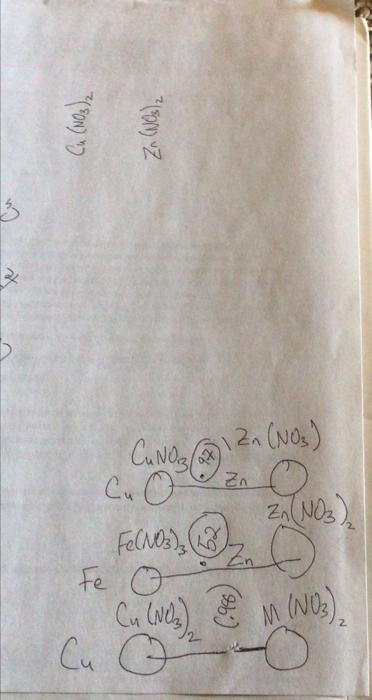
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


