Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question Format: Long Three common ores of iron are FeO, F e 2 O 3 , and F e 3 O 4 . Iron ores
Question Format: Long
Three common ores of iron are FeO, and Iron ores are often reduced to elemental iron by heating in the presence of carbon monoxide. These iron ores are all brittle and do not conduct electricity in their solid form.
a Identify the oxidation number of the iron atoms in each of the three ores.
b Choose one of the iron ores and write a balanced chemical equation to show its reduction reaction with carbon monoxide.
c Considering the types of bonding involved, how and why do the properties of the ores differ from those of pure iron?
Carbon steel is an interstitial alloy of iron with a small amount of carbon which has greater strength and density than pure iron but maintains the metallic properties of iron.
d Is the combination of iron with carbon to form steel a chemical or physical change? Explain your answer.
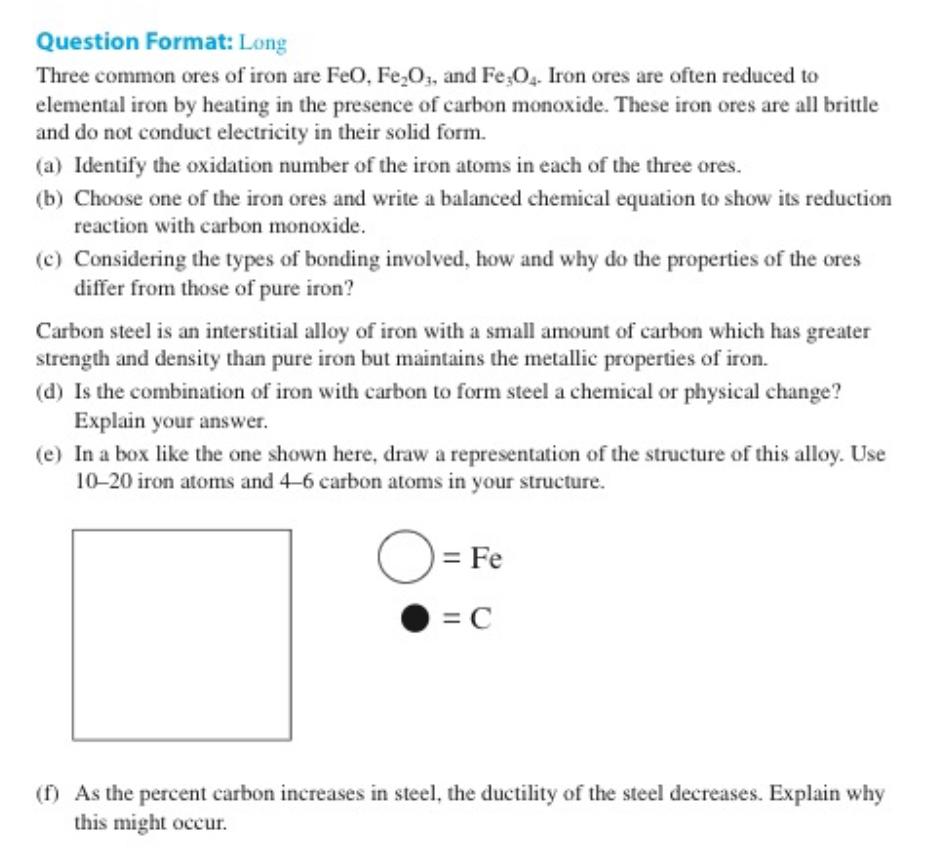
e In a box like the one shown here, draw a representation of the structure of this alloy. Use iron atoms and carbon atoms in your structure.
f As the percent carbon increases in steel, the ductility of the steel decreases. Explain why this might occur.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


