
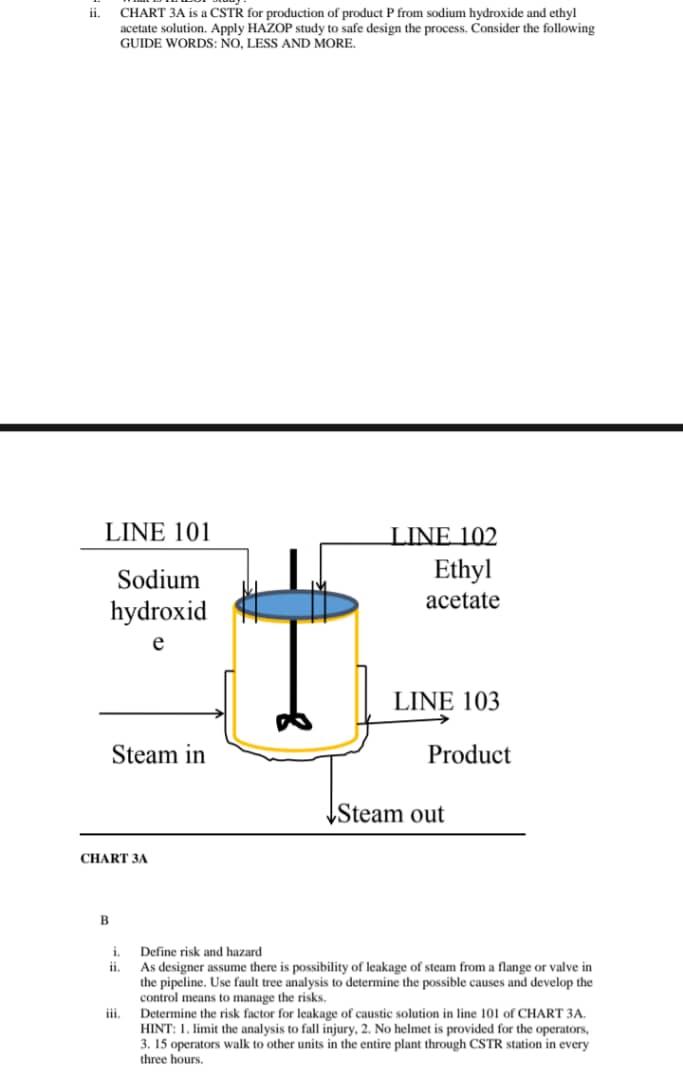


Question Four (23 marks) a. Dilution tank in sulfuric acid plant dilutes oleum with water to produce sulfuric acid. A typical dilution system in the plant requires a vertical shaft centrifugal pump to transport sulfuric acid to its storage vessel, if an installed pump, has mass flow rate, 125kg/hr. Select appropriate PRVs for design and installation to handle a pressure change for outlet blockage of the pump. Justify the answer. b. If the design pressure or MAWP of the tank is 210 psig. Determine the size of a pressure relief valve or dise for the tank: Per ASME BVP Code Sec. VIII D.I, set relief device pressure at 116% of reference pressure. If there is a need to discharge liquid sulfuric acid at 60C to another containment, assume back pressure is more than atmospheric pressure: use 3 times of atmospheric pressure. Hint: stop calculation for viscosity correction factor after determine new value. ii. CHART 3A is a CSTR for production of product P from sodium hydroxide and ethyl acetate solution. Apply HAZOP study to safe design the process. Consider the following GUIDE WORDS: NO, LESS AND MORE. B i. Define risk and hazard ii. As designer assume there is possibility of leakage of steam from a flange or valve in the pipeline. Use fault tree analysis to determine the possible causes and develop the control means to manage the risks. iii. Determine the risk factor for leakage of caustic solution in line 101 of CHART 3A. HINT: 1 . limit the analysis to fall injury, 2. No helmet is provided for the operators, 3. 15 operators walk to other units in the entire plant through CSTR station in every three hours. Question Three (40 marks) Figure 3A shows a flowsheet of first commercial ammonia production from hydrogen and nitrogen molecules. The process is based on Haber-Bosch technology which was purchased by BASF. Hydrogen and nitrogen of ratio 3:1 by volume enter the reactor where iron catalyst aids to produce ammonia. The reaction mixture passes through a condenser where ammonia is separated from the unreacted hydrogen and nitrogen gases. On each pass only about 15% conversion occurs, but any unreacted gases are recycled, and eventually an overall conversion of 97% is achieved. The reaction is carried out at 400450C and 200 atmospheres. The catalyst itself requires a temperature of at least 400C to be efficient. And the reaction mixture is cooled below the boiling point of ammonia (33C). Figure 3A: This is a simplified flowsheet of the first commercial ammonia plant by BASF a. Draw (BLOCK FLOW DIAGRAM) iron catalytic reactor in Figure 3A, and indicate the inlet and out flows-temperature and pressure, components and composition/ratio. Carry out process hazard analysis using hazop to identify process hazards and provide effective controls using layers of process safety - establish deviations by using guide words such as more and less flowrate of the inlet stream. Hint: state causes, consequences and controls b. Carefully examine the Haber-Bosch process in Figure 3A and identify all equipment that require pressure relief valve. State at least one (1) scenario or event that can lead to overpressure in the equipment A gasoline surge drum has capacity 4m3(1060gal) and is normally operated 50% full at 40C (100F) under 20bar absolute pressure ( 280psig ) of hydrogen in the head space and using a level controlled outflow as shown in CHART 4B. Gasoline of specific gravity 0.7 is pumped into the surge drum at a normal flow rate of 130m3/h. assuming the vessel has a hemispherical head and its diameter is 1.12m. if the heat of vaporization of gasoline is 180BTU/lb. i. Determine the relief load for external fire case ii. Determine the discharge area of the relief valve Equations and Data Aw=2D2 wf=Hwap21000FqAwx Assumptions: 1. The vessel is not insulated 2. The relieving temperature is 60C(333K) 3. The vent stack discharges at atmospheric pressure( 1 bar) 4. Z=1.02 for hydrogen










