Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(d) A solution of [Ni(HO)]+ is green and paramagnetic (u =2.90 BM), whereas a solution of [Ni(CN)4] is colorless and diamagnetic. Give a qualitative
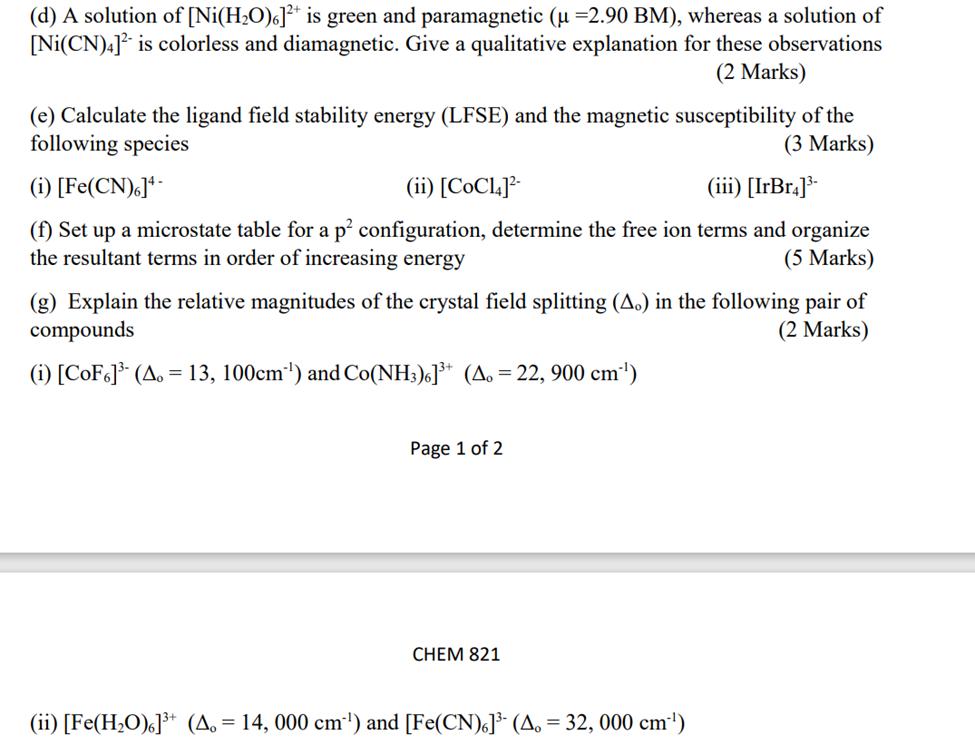
(d) A solution of [Ni(HO)]+ is green and paramagnetic (u =2.90 BM), whereas a solution of [Ni(CN)4] is colorless and diamagnetic. Give a qualitative explanation for these observations (2 Marks) (e) Calculate the ligand field stability energy (LFSE) and the magnetic susceptibility of the following species (3 Marks) (i) [Fe(CN)6]4- (ii) [CoC14]- (iii) [IrBr4]- (f) Set up a microstate table for a p configuration, determine the free ion terms and organize the resultant terms in order of increasing energy (5 Marks) (g) Explain the relative magnitudes of the crystal field splitting (A.) in the following pair of compounds (2 Marks) (i) [CoF6] (A. = 13, 100cm) and Co(NH3)6]+ (A. = 22, 900 cm) Page 1 of 2 CHEM 821 (ii) [Fe(HO)]+ (A. = 14, 000 cm) and [Fe(CN)6] (A. = 32, 000 cm)
Step by Step Solution
★★★★★
3.47 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below d The green color of NiH2O62 is due to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


