Answered step by step
Verified Expert Solution
Question
1 Approved Answer
QUESTIONS 1. Write a mechanism for the reduction of vanillin by sodium borohydride under the reaction conditions you used. action: Weigh 6.5mmol of vanillin into
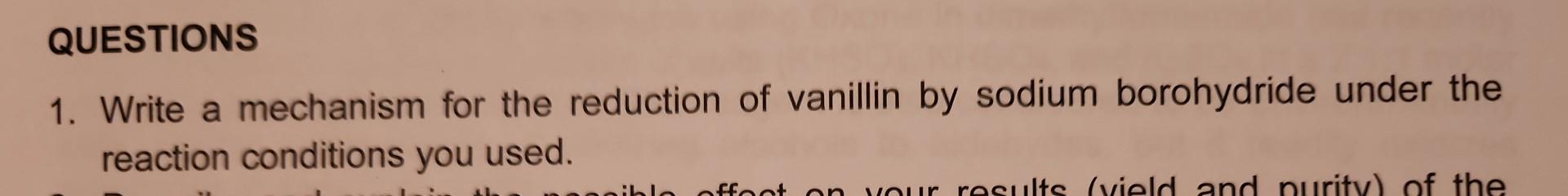

QUESTIONS 1. Write a mechanism for the reduction of vanillin by sodium borohydride under the reaction conditions you used. action: Weigh 6.5mmol of vanillin into a 10mL round bottom flask. Add 2.0mL of ethanol to the flask. Add the stir bar and dissolve the vanillin. If the vanillin does not completely dissolve, gently warm the flask with your hand In a separate reaction vial, dissolve 6.5mmol=O.248NaBH in 1.9mL of 1MNaOH. Cool the round bottom flask containing the vanillin solution in an ice bath. Using a glass pipet, SLOWLY add the NaBH4 solution DROPWISE to the vanillin solution over a period of 5-10 minutes. This is an exothermic reaction, so be careful to add the NaBH4 solution slowly. The reaction temperature should be kept below 25C. After the addition is complete, remove the ice bath and stir the resulting mixture for 10 minutes at room temperature. You should to check the progress of the reaction using TLC (see below). Once the reaction appears complete by TLC, cool the reaction in an ice bath and add 6 MHCl dropwise with stirring until H2 gas is no longer evolved. Check the pH to verify it is acidic. Continue to cool with stirring in the ice bath for 10 minutes. A precipitate will form
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


