questions 2,3 and 6 posted are continued in the next picture.
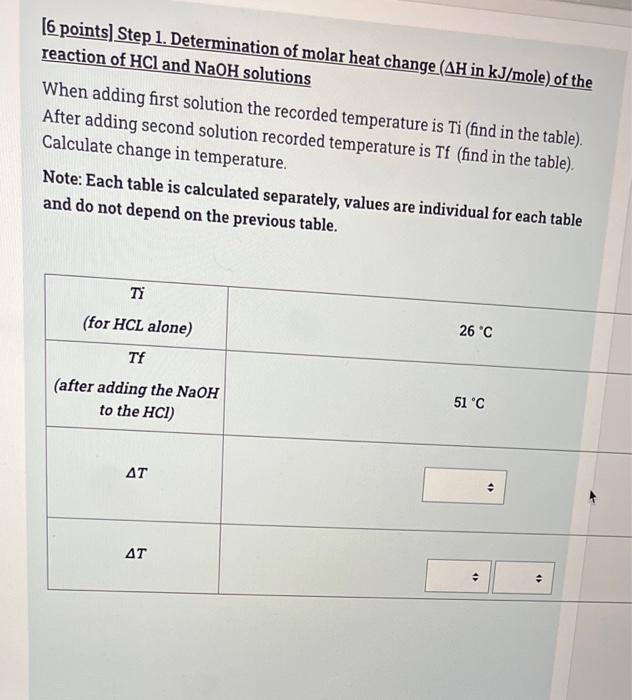
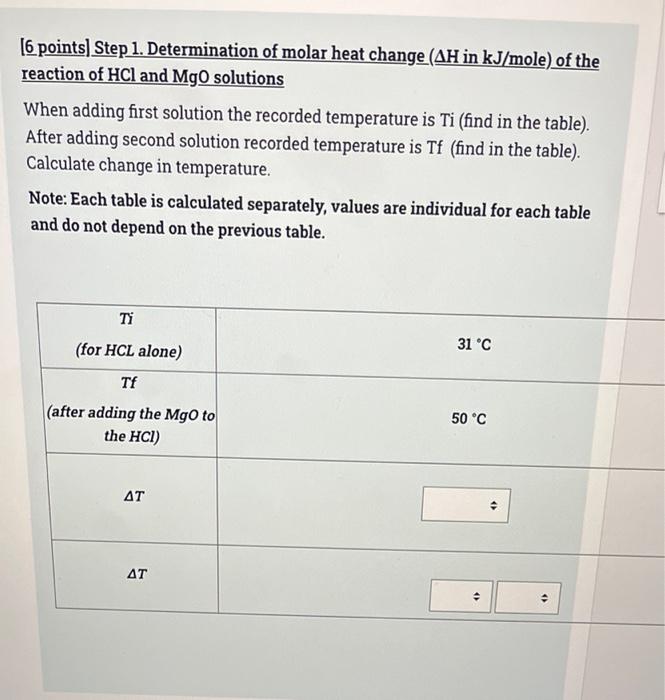
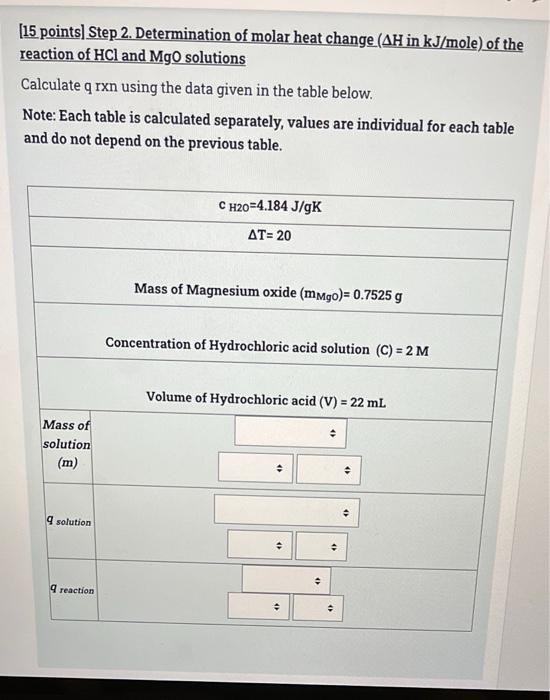
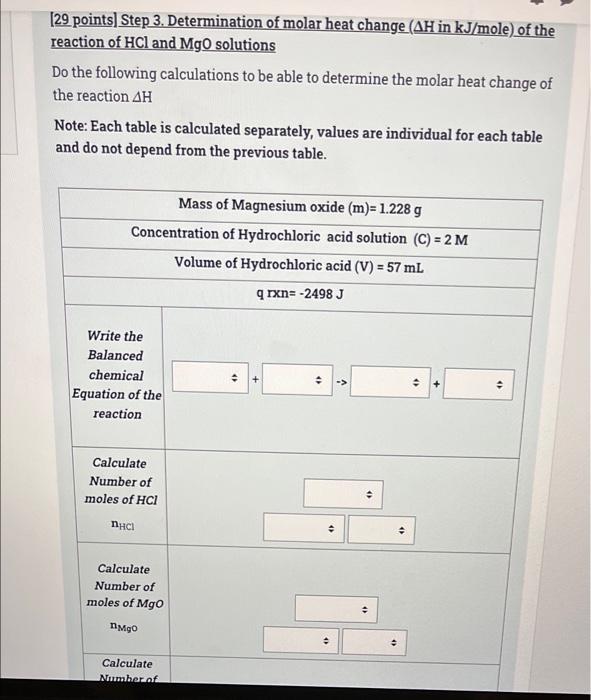
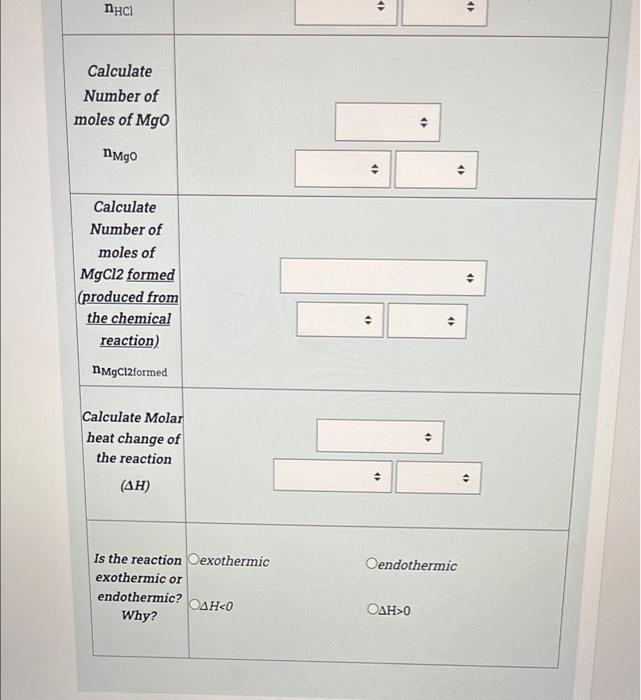
(6 points) Step 1. Determination of molar heat change (AH in kJ/mole) of the reaction of HCl and NaOH solutions When adding first solution the recorded temperature is Ti (find in the table) After adding second solution recorded temperature is Tf (find in the table) Calculate change in temperature, Note: Each table is calculated separately, values are individual for each table and do not depend on the previous table. Ti 26 C (for HCL alone) Tf (after adding the NaOH to the HCI) 51 C AT . . . 2 [15 points) Step 2. Determination of molar heat change (AH in kJ/mole) of the reaction of HCl and NaOH solutions Calculate q rxn using the data given in the table below. Note: Each table is calculated separately, values are individual for each table and do not depend on the previous table. CH20=4.184 J/g.K AT=1 Concentration of Sodium hydroxide solution (C1)= 1 M Volume of Sodium hydroxide solution (V1)= 33 mL Concentration of Hydrochloric acid solution (C2) = 2 M Volume of Hydrochloric acid solution (V2)= 50 mL 4 . Mass of solution . . 4) . solution . . . AT=1 Concentration of Sodium hydroxide solution (C1)=1 M Volume of Sodium hydroxide solution (V1)= 33 mL Concentration of Hydrochloric acid solution (C2) = 2 M Volume of Hydrochloric acid solution (V2)= 50 mL Mass of solution > . q solution . . > 9 reaction . 3 (29 points) Step 3. Determination of molar heat change (AH in kJ/mole) of the reaction of HCl and NaOH solutions Do the following calculations to be able to determine the molar heat change of the reaction AH Note: Each table is calculated separately, values are individual for each table and do not depend from the previous table. Concentration of Sodium hydroxide solution (C1) = 0.03253 M Volume of Sodium hydroxide solution (V1) = 21 mL Concentration of Hydrochloric acid solution (C2) = 1.006 M Volume of Hydrochloric acid solution (V2) = 59 mL q rxn=-576.5J Write the Balanced chemical Equation of the reaction . + D D Calculate Number of moles of NaOH . 4 nNaOH Calculate Number of moles of HCI Calculate Number of moles of H20 formed (produced from the chemical reaction) nh20 formed . Calculate Molar heat change of the reaction (AH) . . Is the reaction exothermic endothermic or endothermic? 0 MacBook Pro [6 points) Step 1. Determination of molar heat change (AH in kJ/mole) of the reaction of HCl and MgO solutions When adding first solution the recorded temperature is Ti (find in the table). After adding second solution recorded temperature is Tf (find in the table). Calculate change in temperature. Note: Each table is calculated separately, values are individual for each table and do not depend on the previous table. Ti 31 C (for HCL alone) Tf (after adding the Mgo to the HCI) 50C AT > AT . . (15 points) Step 2. Determination of molar heat change (AH in kJ/mole) of the reaction of HCl and MgO solutions Calculate q rxn using the data given in the table below. Note: Each table is calculated separately, values are individual for each table and do not depend on the previous table. C H20=4.184 J/gK AT= 20 Mass of Magnesium oxide (mMgo)= 0.7525 g Concentration of Hydrochloric acid solution (C) = 2 M Volume of Hydrochloric acid (V) = 22 mL . Mass of solution (m) . . 9 solution . + 4 9 reaction . . (29 points) Step 3. Determination of molar heat change (AH in kJ/mole) of the reaction of HCl and MgO solutions Do the following calculations to be able to determine the molar heat change of the reaction AH Note: Each table is calculated separately, values are individual for each table and do not depend from the previous table. Mass of Magnesium oxide (m)=1.228 g Concentration of Hydrochloric acid solution (C) = 2 M Volume of Hydrochloric acid (V) = 57 mL q rxn=-2498 J Write the Balanced chemical Equation of the reaction . . -> A . Calculate Number of moles of HCI . > . Calculate Number of moles of Mgo . NMgo 4) Calculate Numberat 1 . Calculate Number of moles of Mgo . NMgo . Calculate Number of moles of MgCl2 formed (produced from the chemical reaction) nMgCl2formed . . . Calculate Molar heat change of the reaction () . Oendothermic Is the reaction exothermic exothermic or endothermic? 0