Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Questions and training MCQ: 1. Which of the following transitions is the highest energy transition? (a) n to (b) n to (c) to (d) to
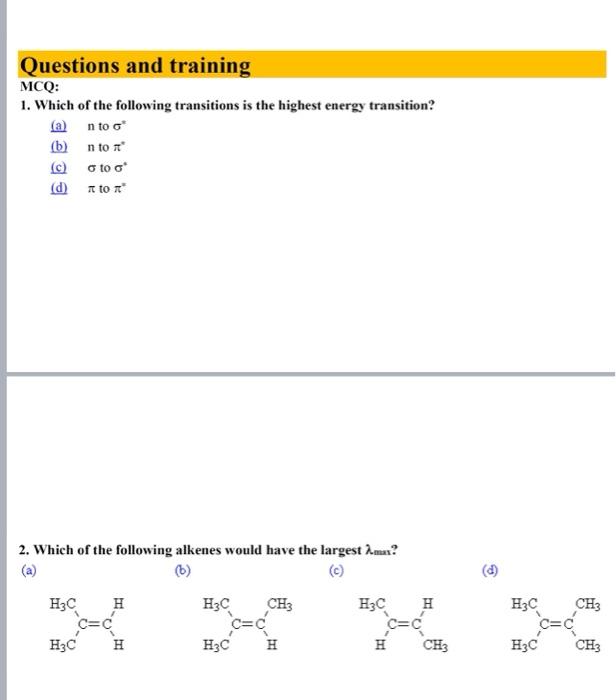
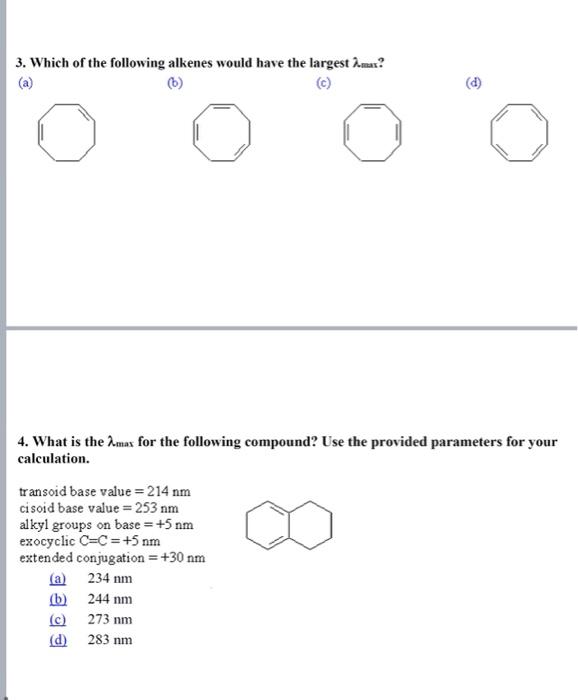
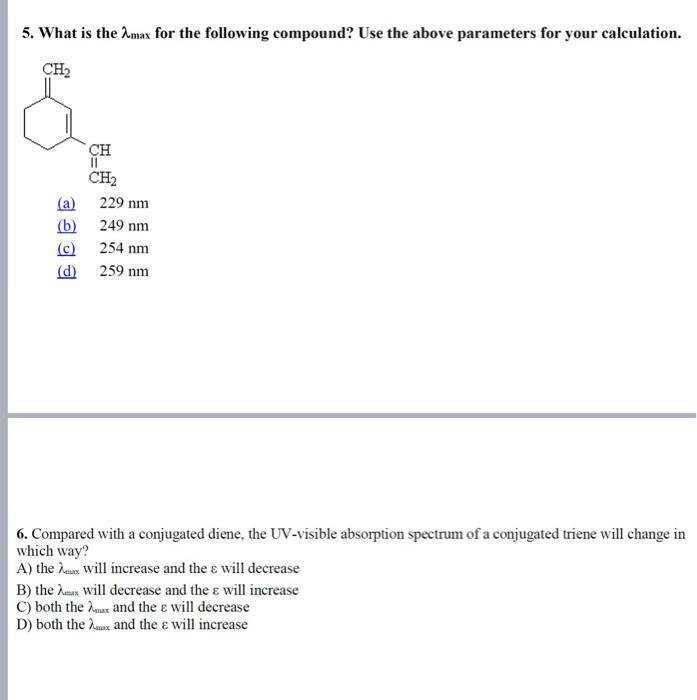
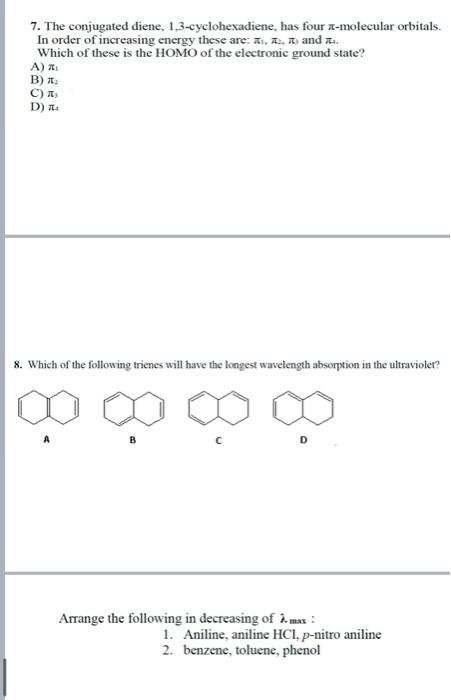 Questions and training MCQ: 1. Which of the following transitions is the highest energy transition? (a) n to (b) n to (c) to (d) to 2. Which of the following alkenes would have the largest max ? 3. Which of the following alkenes would have the largest max ? (a) (b) (c) (d) 4. What is the max for the following compound? Use the provided parameters for your calculation. transoid base value =214nm cisoid base value =253nm alkyl groups on base =+5nm exocyclic C=C=+5nm extended conjugation =+30nm (a) 234nm (b) 244nm (c) 273nm (d) 283nm 5. What is the max for the following compound? Use the above parameters for your calculation. (a) 229nm (b) 249nm (c) 254nm (d) 259nm 6. Compared with a conjugated diene, the UV-visible absorption spectrum of a conjugated triene will change in which way? A) the max will increase and the will decrease B) the max will decrease and the will increase C) both the max and the will decrease D) both the max and the will increase 7. The conjugated diene, 1,3-cyclohexadiene, has four -molecular orbitals. In order of increasing energy these are: 1,2,0 and 2. Which of these is the HOMO of the electronic ground state? A) 1 B) 2 C) 3 D) 4 8. Which of the following trienes will have the longest wavelength absorption in the ultraviolet? A B C D Arrange the following in decreasing of max : 1. Aniline, aniline HCl,p-nitro aniline 2. benzene, toluene, phenol
Questions and training MCQ: 1. Which of the following transitions is the highest energy transition? (a) n to (b) n to (c) to (d) to 2. Which of the following alkenes would have the largest max ? 3. Which of the following alkenes would have the largest max ? (a) (b) (c) (d) 4. What is the max for the following compound? Use the provided parameters for your calculation. transoid base value =214nm cisoid base value =253nm alkyl groups on base =+5nm exocyclic C=C=+5nm extended conjugation =+30nm (a) 234nm (b) 244nm (c) 273nm (d) 283nm 5. What is the max for the following compound? Use the above parameters for your calculation. (a) 229nm (b) 249nm (c) 254nm (d) 259nm 6. Compared with a conjugated diene, the UV-visible absorption spectrum of a conjugated triene will change in which way? A) the max will increase and the will decrease B) the max will decrease and the will increase C) both the max and the will decrease D) both the max and the will increase 7. The conjugated diene, 1,3-cyclohexadiene, has four -molecular orbitals. In order of increasing energy these are: 1,2,0 and 2. Which of these is the HOMO of the electronic ground state? A) 1 B) 2 C) 3 D) 4 8. Which of the following trienes will have the longest wavelength absorption in the ultraviolet? A B C D Arrange the following in decreasing of max : 1. Aniline, aniline HCl,p-nitro aniline 2. benzene, toluene, phenol




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


