Questions are last 2 pictures. PLEASE HELP ON PRELAB WILL UPVOTE PLEASE!




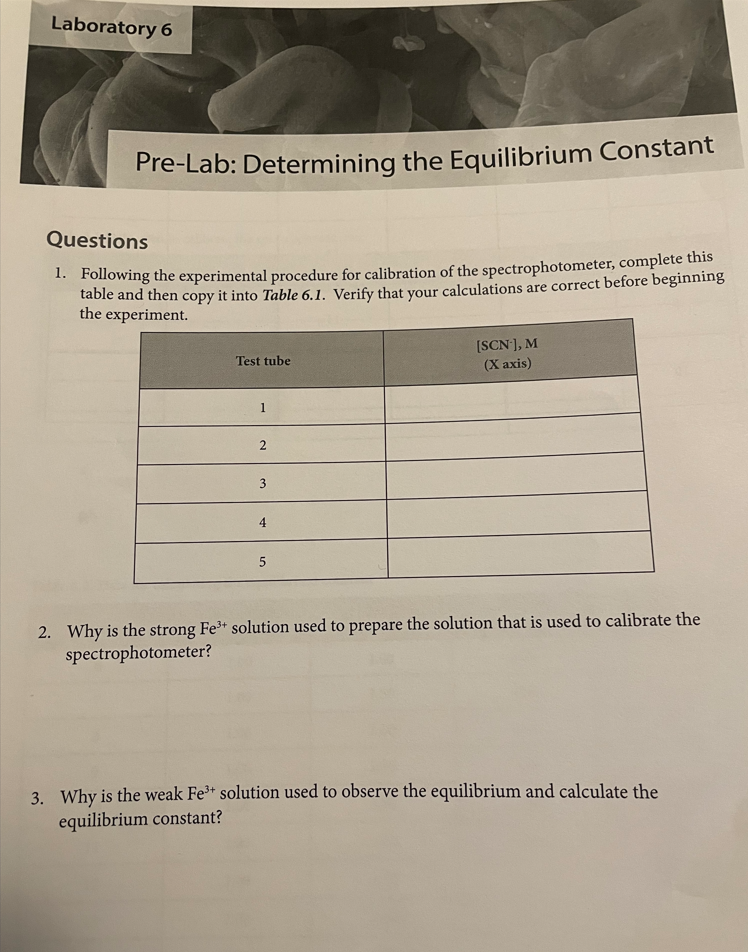
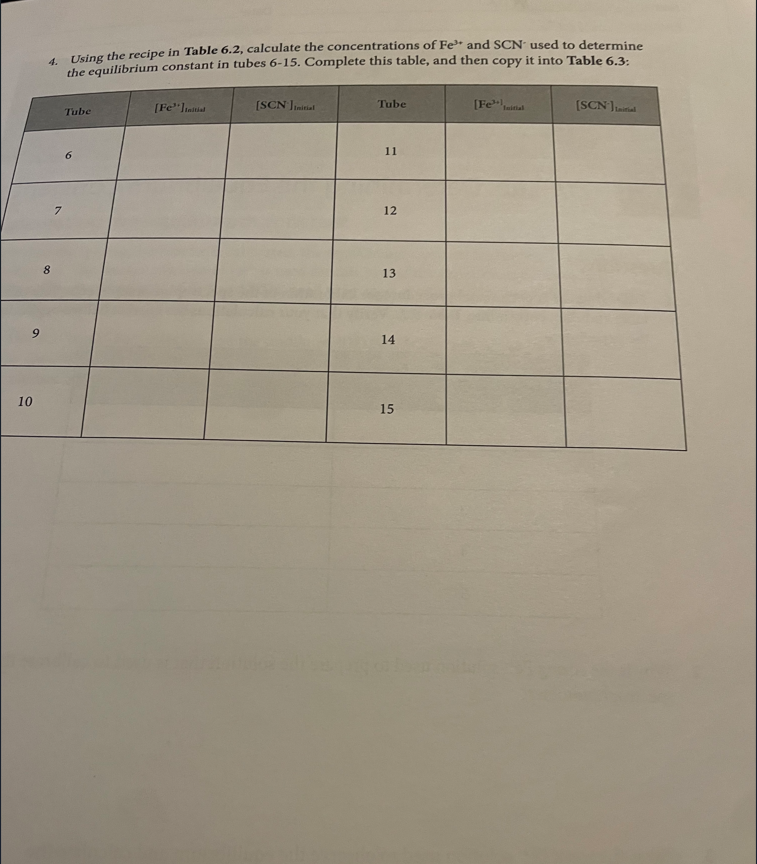
Introduction Many chemical reactions proceed in the forward direction as well as in the reverse direction until they reach a condition of dynamic equilibrium, in which no further progress is observable in either direction. In this lab, we will observe the formation of the Fe(SCN)2+ or "thiocyanatoiron (III)" complex ion. It forms when Fe3+ (which is in solution as a nitrate salt) and SCN(which is in solution as a potassium salt) are mixed together: Fe3+(aq)+SCN(aq)Fe(SCN)2+ You will measure the concentration of Fe(SCN)2+ using a spectrophotometer, which measures how much visible light is absorbed by a colorful solution at a certain wavelength. Fe(SCN)2+ absorbs 450nm violet light, making it appear yellow-orange to the observer. There is a linear relationship between the molar concentration of a colorful sample and the amount of light it absorbs. Let "A" be the absorbance of the solution as measured by the spectrophotometer, and " C " be the molar concentration of the sample: A=C The relationship between absorbance and concentration is known as the Beer-Lambert Law, or more simply Beer's Law. The molar absorptivity or molar extinction coefficient, denoted by the Greek letter epsilon () is the slope of the line determined by plotting absorbance v s. molar concentration. The concentration of an unknown is calculated from its absorbance using its determined molar absorptivity: A=C Rearranges to C=A/ To measure the molar absorptivity, the spectrophotometer must be calibrated by placing known concentrations of the colorful substance into the spectrophotometer and observing the instrument's response. To make solutions with known concentrations of Fe(SCN)2, a very high concentration of Fe" and a very low concentration of SCN. effectively becomes a limiting reagent. The concentration of formation of Fe(SCN)2+ and solutions is the same as the concentration of SCN ' in each test tube, because all of the SCN. reacts with the Fe3 to form the complex. Once the instrument has been calibrated, comparable concentrations of the reactants will be mixed together to find how much of the complex is produced and to determine the equilibrium constant for the reaction. Unlike rate constants, equilibrium constant expressions can be derived directly using a reaction's balanced chemical equation. Equilibrium constants are exponentially related to the number of reactant and product molecules involved in the reaction, according to the Law of Mass Action: For any reaction aA+bBcC+dD The expression for the equilibrium constant is K=[A]a[B]b[C]c[D]d In this experiment, you will be calculating the equilibrium constant for production of Fe(SCN)2+ : Fe3+(aq)+SCN(aq)Fe(SCN)2+ The equilibrium constant will be calculated using the Law of Mass Action as follows: K=[Fe3+][SCN][Fe(SCN)2+] The concentration of the Fe(SCN)2+ complex is determined directly by the calibrated spectrophotometer, using Beer's law with a molar absorptivity calculated from the calibration curve. Comparable concentrations of Fe3+ and SCNare used so the equilibrium doesn't strongly favor the reactant or product side and it's observable, given the value of the equilibrium constant. Since each mole Fe(SCN)2+ is made from one mole of Fe3+ and one mole of SCN, the equilibrium concentrations of Fe3+ and SCNare each their initial concentration (which is the concentration in the test tube after dilution according to the directions) minus the concentration of Fe(SCN)2+ that formed. Procedure Obtaining the reagents Obtain the following reagents in appropriately sized and labeled beakers: 1. 30mL0.0025MKSCN 2. 30mL0.25MFe(NO3)3 (This solution is orange from the high concentration of Fe3+ ) 3. 20mL0.0025MFe(NO3), (This solution is colorless because the concentration of Fe3+ is low) 4. 50mL0.1MHNO3 (Used to dilute test tubes containing Fe3+ to a uniform volume) Calibrating the spectrophotometer Calibrate the spectrophotometer by making known concentrations of the Fe(SCN)2+ complex that have a linear increase in concentration. Plotting absorbance (measured by the spectrophotometer) vs. concentration will give a slope which is the molar absorptivity. The concentrations of unknowns can be determined using Beer's law: since A=C, that means that for any unknown C=A/ A very strong solution of Fe3+ and a very weak solution of SCN* will be used to calibrate the spectrophotometer. There will be so much Fe3+ relative to the amount of SCNthat the equilibrium will be forced all the way to the right. That means that SCNacts as a limiting reagent so the concentration of Fe(SCN)2+ is the same as the concentration of SCN. 1. Determine whether the pipettes that you will be using are Mohr or serological. If the pipette is a Mohr pipette, then it can only be used within the part of the pipette that is graduated; NOT all the way to the tip. 2. Use a pipette to deliver 4.00mL of 0.0025MKSCN into a 100mL graduated cylinder. Dilute to exactly 100mL. Pour this solution into a beaker to ensure thorough mixing before using it. 3. Use a pipette to deliver 5.00mL of 0.25MFe(NO3)3 into each of five large numbered test tubes 4. To the five test tubes, pipette the following solutions to make each test tube have a total volume of 10.00mL : 1. 1.00mL dilute KSCN+4.00mL0.1MHNO3 2. 2.00mL dilute KSCN+3.00mL0.1MHNO3 3. 3.00mL dilute KSCN+2.00mL0.1MHNO3 4. 4.00mL dilute KSCN+1.00mL0.1MHNO3 5. 5.00mL dilute KSCN 5. Turn the knob on the spectrophotometer to adjust the wavelength to 450nm. Place a cuvette 3/4 filled with water in the sample holder and press the button that says " 0.00." When the instrument is finished auto-zeroing, mix each of your test tubes thoroughly by swirling, place a sample of each in a separate cuvette, and place it in the instrument to read its absorbance. Record absorbance on the table in the next step. 6. According to the procedure, you just diluted 4.00mL of 0.0025MKSC to a total volume of 100mL, added a given volume (1,2,3,4, and 5mL) of the diluted KSCN to test tubes, and diluted it to a total volume of 10.00mL. Calculate the concentration of SCNin each test tube and record it in Table 6.1 Record your sample calculations in the space below the table. 7. Use a spreadsheet program such as Excel to plot concentration on the X axis and absorbance on the Y axis. You should force the Y intercept to 0 , since you zeroed the spectrophotometer with water which does not absorb visible light. Display the equation and the R2 value. Attach your plot to your lab report, and give the molar absorptivity (slope) and R2 value in the spaces below Table 6.1. Calculating the equilibrium constant Now that the spectrophotometer is calibrated, the equilibrium constant for the reaction can be determined. The weaker solution of Fe3+ is used for this part of the experiment because now favor the reactants or products. 8. Use the "weak" Fe3+ solution and the undiluted 0.0025M SCN-solution to make the mixtures in Table 6.2 in large test tubes, and record the absorbance of each solution in the table. All volumes are in mL and should be delivered by pipette. rre-Lab: vetermnng Questions 1. Following the experimental procedure for calibration of the spectrophotometer, complete this table and then copy it into Table 6.1. Verify that your calculations are correct before beginning the experiment. 2. Why is the strong Fe3+ solution used to prepare the solution that is used to calibrate the spectrophotometer? 3. Why is the weak Fe3+ solution used to observe the equilibrium and calculate the equilibrium constant? 4. Using the recipe in Table 6.2, calculate the concentrations of Fe3+ and SCNused to determine the equilibrium constant in tubes 6-15. Complete this table, and then copy it into Table 6.3: Introduction Many chemical reactions proceed in the forward direction as well as in the reverse direction until they reach a condition of dynamic equilibrium, in which no further progress is observable in either direction. In this lab, we will observe the formation of the Fe(SCN)2+ or "thiocyanatoiron (III)" complex ion. It forms when Fe3+ (which is in solution as a nitrate salt) and SCN(which is in solution as a potassium salt) are mixed together: Fe3+(aq)+SCN(aq)Fe(SCN)2+ You will measure the concentration of Fe(SCN)2+ using a spectrophotometer, which measures how much visible light is absorbed by a colorful solution at a certain wavelength. Fe(SCN)2+ absorbs 450nm violet light, making it appear yellow-orange to the observer. There is a linear relationship between the molar concentration of a colorful sample and the amount of light it absorbs. Let "A" be the absorbance of the solution as measured by the spectrophotometer, and " C " be the molar concentration of the sample: A=C The relationship between absorbance and concentration is known as the Beer-Lambert Law, or more simply Beer's Law. The molar absorptivity or molar extinction coefficient, denoted by the Greek letter epsilon () is the slope of the line determined by plotting absorbance v s. molar concentration. The concentration of an unknown is calculated from its absorbance using its determined molar absorptivity: A=C Rearranges to C=A/ To measure the molar absorptivity, the spectrophotometer must be calibrated by placing known concentrations of the colorful substance into the spectrophotometer and observing the instrument's response. To make solutions with known concentrations of Fe(SCN)2, a very high concentration of Fe" and a very low concentration of SCN. effectively becomes a limiting reagent. The concentration of formation of Fe(SCN)2+ and solutions is the same as the concentration of SCN ' in each test tube, because all of the SCN. reacts with the Fe3 to form the complex. Once the instrument has been calibrated, comparable concentrations of the reactants will be mixed together to find how much of the complex is produced and to determine the equilibrium constant for the reaction. Unlike rate constants, equilibrium constant expressions can be derived directly using a reaction's balanced chemical equation. Equilibrium constants are exponentially related to the number of reactant and product molecules involved in the reaction, according to the Law of Mass Action: For any reaction aA+bBcC+dD The expression for the equilibrium constant is K=[A]a[B]b[C]c[D]d In this experiment, you will be calculating the equilibrium constant for production of Fe(SCN)2+ : Fe3+(aq)+SCN(aq)Fe(SCN)2+ The equilibrium constant will be calculated using the Law of Mass Action as follows: K=[Fe3+][SCN][Fe(SCN)2+] The concentration of the Fe(SCN)2+ complex is determined directly by the calibrated spectrophotometer, using Beer's law with a molar absorptivity calculated from the calibration curve. Comparable concentrations of Fe3+ and SCNare used so the equilibrium doesn't strongly favor the reactant or product side and it's observable, given the value of the equilibrium constant. Since each mole Fe(SCN)2+ is made from one mole of Fe3+ and one mole of SCN, the equilibrium concentrations of Fe3+ and SCNare each their initial concentration (which is the concentration in the test tube after dilution according to the directions) minus the concentration of Fe(SCN)2+ that formed. Procedure Obtaining the reagents Obtain the following reagents in appropriately sized and labeled beakers: 1. 30mL0.0025MKSCN 2. 30mL0.25MFe(NO3)3 (This solution is orange from the high concentration of Fe3+ ) 3. 20mL0.0025MFe(NO3), (This solution is colorless because the concentration of Fe3+ is low) 4. 50mL0.1MHNO3 (Used to dilute test tubes containing Fe3+ to a uniform volume) Calibrating the spectrophotometer Calibrate the spectrophotometer by making known concentrations of the Fe(SCN)2+ complex that have a linear increase in concentration. Plotting absorbance (measured by the spectrophotometer) vs. concentration will give a slope which is the molar absorptivity. The concentrations of unknowns can be determined using Beer's law: since A=C, that means that for any unknown C=A/ A very strong solution of Fe3+ and a very weak solution of SCN* will be used to calibrate the spectrophotometer. There will be so much Fe3+ relative to the amount of SCNthat the equilibrium will be forced all the way to the right. That means that SCNacts as a limiting reagent so the concentration of Fe(SCN)2+ is the same as the concentration of SCN. 1. Determine whether the pipettes that you will be using are Mohr or serological. If the pipette is a Mohr pipette, then it can only be used within the part of the pipette that is graduated; NOT all the way to the tip. 2. Use a pipette to deliver 4.00mL of 0.0025MKSCN into a 100mL graduated cylinder. Dilute to exactly 100mL. Pour this solution into a beaker to ensure thorough mixing before using it. 3. Use a pipette to deliver 5.00mL of 0.25MFe(NO3)3 into each of five large numbered test tubes 4. To the five test tubes, pipette the following solutions to make each test tube have a total volume of 10.00mL : 1. 1.00mL dilute KSCN+4.00mL0.1MHNO3 2. 2.00mL dilute KSCN+3.00mL0.1MHNO3 3. 3.00mL dilute KSCN+2.00mL0.1MHNO3 4. 4.00mL dilute KSCN+1.00mL0.1MHNO3 5. 5.00mL dilute KSCN 5. Turn the knob on the spectrophotometer to adjust the wavelength to 450nm. Place a cuvette 3/4 filled with water in the sample holder and press the button that says " 0.00." When the instrument is finished auto-zeroing, mix each of your test tubes thoroughly by swirling, place a sample of each in a separate cuvette, and place it in the instrument to read its absorbance. Record absorbance on the table in the next step. 6. According to the procedure, you just diluted 4.00mL of 0.0025MKSC to a total volume of 100mL, added a given volume (1,2,3,4, and 5mL) of the diluted KSCN to test tubes, and diluted it to a total volume of 10.00mL. Calculate the concentration of SCNin each test tube and record it in Table 6.1 Record your sample calculations in the space below the table. 7. Use a spreadsheet program such as Excel to plot concentration on the X axis and absorbance on the Y axis. You should force the Y intercept to 0 , since you zeroed the spectrophotometer with water which does not absorb visible light. Display the equation and the R2 value. Attach your plot to your lab report, and give the molar absorptivity (slope) and R2 value in the spaces below Table 6.1. Calculating the equilibrium constant Now that the spectrophotometer is calibrated, the equilibrium constant for the reaction can be determined. The weaker solution of Fe3+ is used for this part of the experiment because now favor the reactants or products. 8. Use the "weak" Fe3+ solution and the undiluted 0.0025M SCN-solution to make the mixtures in Table 6.2 in large test tubes, and record the absorbance of each solution in the table. All volumes are in mL and should be delivered by pipette. rre-Lab: vetermnng Questions 1. Following the experimental procedure for calibration of the spectrophotometer, complete this table and then copy it into Table 6.1. Verify that your calculations are correct before beginning the experiment. 2. Why is the strong Fe3+ solution used to prepare the solution that is used to calibrate the spectrophotometer? 3. Why is the weak Fe3+ solution used to observe the equilibrium and calculate the equilibrium constant? 4. Using the recipe in Table 6.2, calculate the concentrations of Fe3+ and SCNused to determine the equilibrium constant in tubes 6-15. Complete this table, and then copy it into Table 6.3












