Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The densities of aqueous solutions of copper (II) sulfate at 20 C were measured as set out below. a. Plot the mol of CUSO4
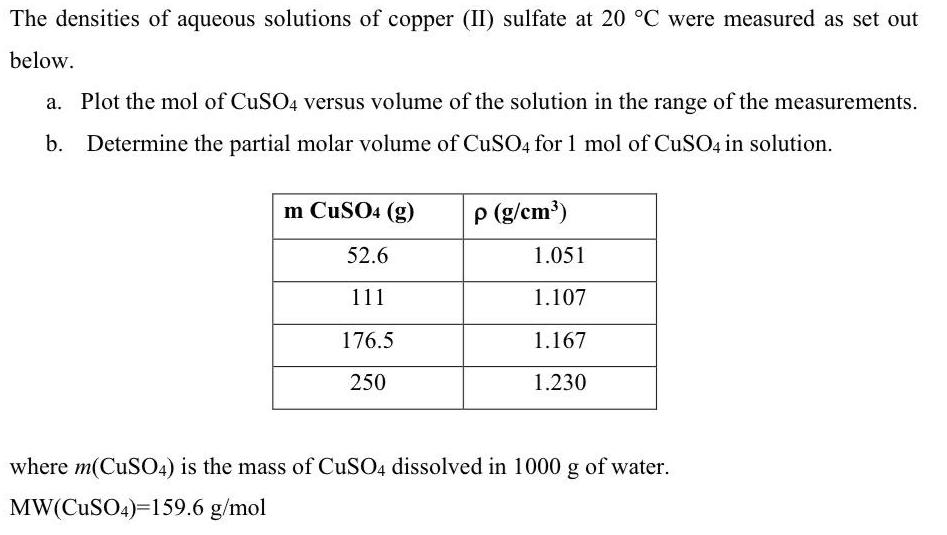
The densities of aqueous solutions of copper (II) sulfate at 20 C were measured as set out below. a. Plot the mol of CUSO4 versus volume of the solution in the range of the measurements. b. Determine the partial molar volume of CuSO4 for 1 mol of CuSO4 in solution. m CuSO4 (g) p (g/cm') 52.6 1.051 111 1.107 176.5 1.167 250 1.230 where m(CUSO4) is the mass of CUSO4 dissolved in 1000 g of water. MW(CuSO4)=159.6 g/mol
Step by Step Solution
★★★★★
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60915c5db143b_209834.pdf
180 KBs PDF File
60915c5db143b_209834.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


