Questions In images

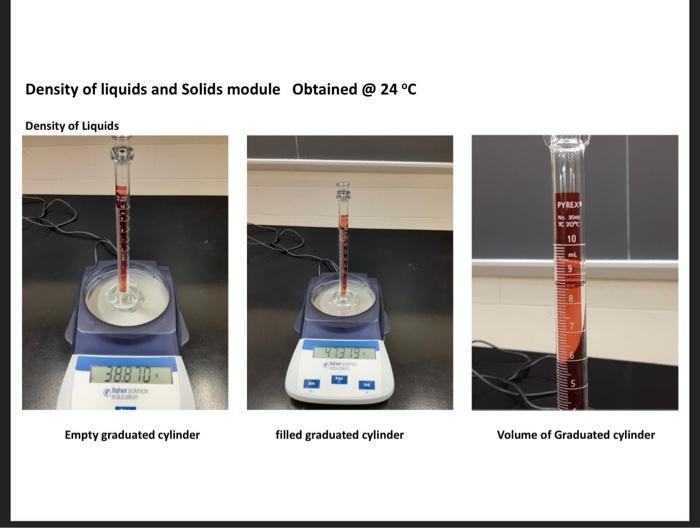
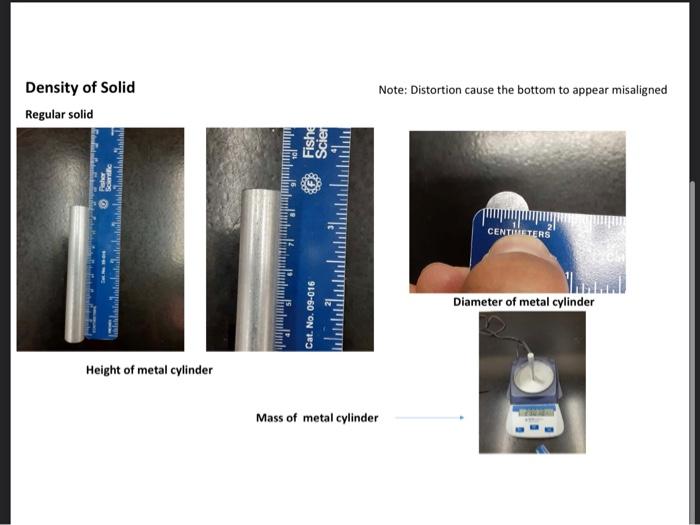
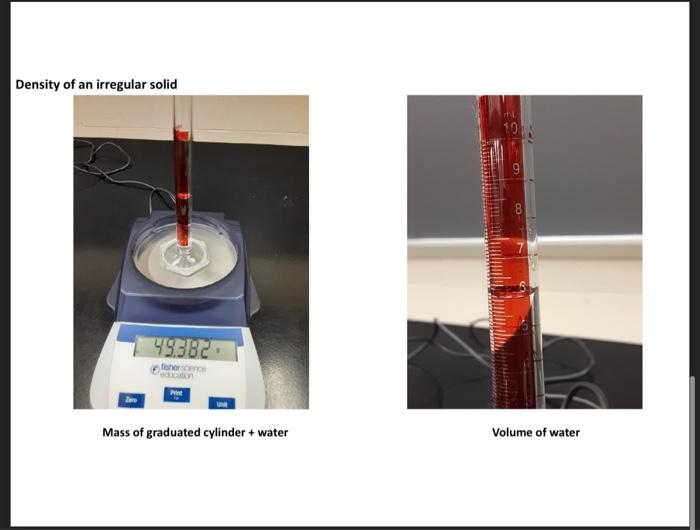
liplitid as the saniple. Recond all mansurements in the dafa section (on prge 29), Note that the gnatiated cylinuler seroes two purposes in the procadure used: (1) as a ortainer to onsentemily weight the liquid samyles. (2) as a devioe for measuring volabne. 1. Weigh a dry, empty 10ml graduated cylinder on a Cent-o-gram balance. Record the weight as accurately as possible. (The last namber shoulf be esthmathe, giving a reading wigh there digits after fhe derinal point.) 2. Pour between 6 and 9mL of the sample into the cylinder. 3. Weigh the cylinder with the sample in it. Record the weight as accurately as possible (The last mamber should be estintated, giving a reading with thine digits after the decienal point.) 4. Record the volume in the cylinder as accurately as possible. (The last inonber should be estimatert, giring a rending with two digits affer the decinal point.) 5. Record the temperature as accurately as possible. (One digit after the decimal point.) 6. Calculate the weight of the sample. (Three digits after the decinal point.) 7. Calculate the density of the sample. (Two digits after the decimal poinf.) 1. Why is it important to necord the temperature at which the density of a sample is measured? 2. List the names of some calabrated laboratory glassware that can be used to measure the volume of liquids. 3. Consider two samples of laquids; Sample (A):3.48mL of mencury (d=13.6g/mL) Sample (B). 60.0mL of alcohot (d=0.789g/mL) Whach sample has the greatest volume? DSample A DSample B DSame Which sample has the greatest density? Sample A DSample B Dame Which sample has the greatest mass? Sample A DSample B DSame 4. Will a 500-gram sample of carbon tetrachloride have a greater density than a 7.0-gram sample of carbon tetrachloride? Explain yout answer. Yes: NO Density of liquids and Solids module Obtained @24 C Densitv of Liauids Empty graduated cylinder filled graduated cylinder Volume of Graduated cylinder Unknown liquid of Solid Note: Distortion cause the bottom to appear misaligned olid Density of an irregular solid Mass of graduated cylinder + water Mass of 10mL graduated + water + irregular solid (iron pellets) New volume of graduated cylinder