Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Questions in pictures given & graphs are included. 50mL of water was used for each item in this experiment. I need help please! 22. Calculate
Questions in pictures given & graphs are included.
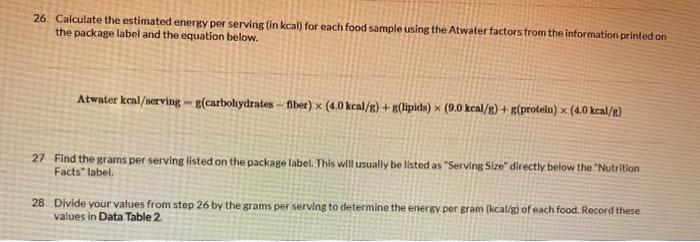
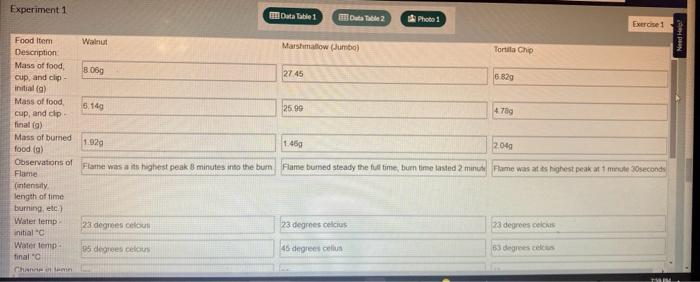
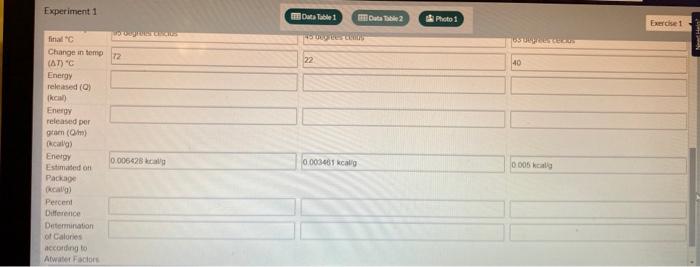
22. Calculate the number of Calories of heat absorbed by the water (Q) for each of the food samples using the equation below and record in Data Table 2. Q=Tmc 23 Calculate the heat per gram produced (in kcal/gram) for each of the food samples using the equation below and record in Data Table 2. 2. MmandQgramheat 24 Observe the food label for each sample burned and determine the energy (heat) per gram (kcal/g) for each food item according to the label. Record these values in Data Table 2. Calculate the percent difference between the published energy per gram found in the previous step and your test results for each food item using the percent difference equation below and record in Data Table 2. PercentDifference(2ExperimentalValue+TheoreticalValue)ExperimentalValue-TheoreticalValue100% 26 Calculate the estimated energy per serving (in kcal) for each food sample using the Atwater factors from the information printed on the package label and the equation below. Atwaterkeal/servingg(carbohydratesfber)(4.0kcal/g)+g(lipids)(9.0kcal/g)+g(protein)(4.0kcal/g) Find the grams per serving listed on the package label. This will usually be listed as "Serving Size" directly below the "Nutrition Facts" labet. 28. Divide your values from step 26 by the grams per serving to determine the energy per gram (kcal/g) of each food. Record these values in Data Table 2. Experiment 1 Experiment 1 22. Calculate the number of Calories of heat absorbed by the water (Q) for each of the food samples using the equation below and record in Data Table 2. Q=Tmc 23 Calculate the heat per gram produced (in kcal/gram) for each of the food samples using the equation below and record in Data Table 2. 2. MmandQgramheat 24 Observe the food label for each sample burned and determine the energy (heat) per gram (kcal/g) for each food item according to the label. Record these values in Data Table 2. Calculate the percent difference between the published energy per gram found in the previous step and your test results for each food item using the percent difference equation below and record in Data Table 2. PercentDifference(2ExperimentalValue+TheoreticalValue)ExperimentalValue-TheoreticalValue100% 26 Calculate the estimated energy per serving (in kcal) for each food sample using the Atwater factors from the information printed on the package label and the equation below. Atwaterkeal/servingg(carbohydratesfber)(4.0kcal/g)+g(lipids)(9.0kcal/g)+g(protein)(4.0kcal/g) Find the grams per serving listed on the package label. This will usually be listed as "Serving Size" directly below the "Nutrition Facts" labet. 28. Divide your values from step 26 by the grams per serving to determine the energy per gram (kcal/g) of each food. Record these values in Data Table 2. Experiment 1 Experiment 1 50mL of water was used for each item in this experiment.
I need help please! 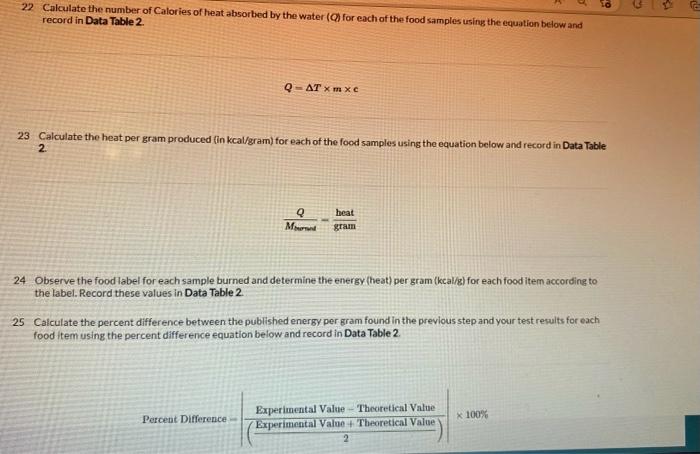




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


