questions needed
part of lab handout
procedure given
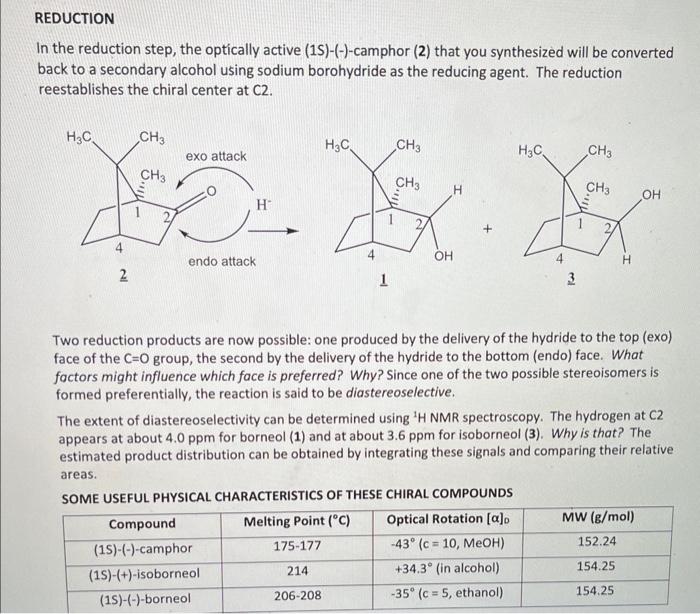
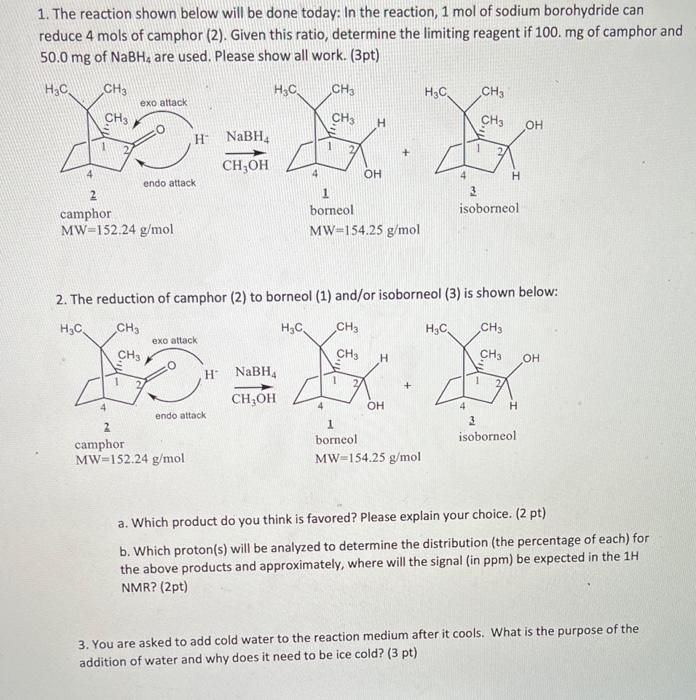
1. The reaction shown below will be done today: In the reaction, 1mol of sodium borohydride can reduce 4 mols of camphor (2). Given this ratio, determine the limiting reagent if 100.mg of camphor and 50.0mg of NaBH4 are used. Please show all work. (3pt) 2. The reduction of camphor (2) to borneol (1) and/or isoborneol (3) is shown below: a. Which product do you think is favored? Please explain your choice. (2pt) b. Which proton(s) will be analyzed to determine the distribution (the percentage of each) for the above products and approximately, where will the signal (in ppm) be expected in the 1H NMR? (2pt) 3. You are asked to add cold water to the reaction medium after it cools. What is the purpose of the addition of water and why does it need to be ice cold? (3 pt) In the reduction step, the optically active (1S)-(-)-camphor (2) that you synthesized will be converted back to a secondary alcohol using sodium borohydride as the reducing agent. The reduction reestablishes the chiral center at C2. Two reduction products are now possible: one produced by the delivery of the hydride to the top (exo) face of the C=O group, the second by the delivery of the hydride to the bottom (endo) face. What factors might influence which face is preferred? Why? Since one of the two possible stereoisomers is formed preferentially, the reaction is said to be diastereoselective. The extent of diastereoselectivity can be determined using 1H NMR spectroscopy. The hydrogen at C2 appears at about 4.0ppm for borneol (1) and at about 3.6ppm for isoborneol (3). Why is that? The estimated product distribution can be obtained by integrating these signals and comparing their relative areas. REDUCTION PROCEDURE 1. Reweigh the flask containing the camphor and record the mass. Based on this mass, calculate the amount of solvent and reducing agent needed for this reaction. To make these calculations: use 0.5mL of methanol solvent for every 100mg of camphor and 50mg of NaBH f for every 100 mg of camphor. Even though 1 mole of NaBH4 can reduce 4 moles of camphor, excess reducing agent will be used. If the TLC of your product indicated a significant amount of starting material was present, obtain 100mg of racemic camphor and use it for the reduction. Or if you isolated less than 100mg of camphor product, obtain enough racemic camphor from the TA to supplement your yield. Remember, this means your starting material is no longer enantiomerically pure. To the flask containing your camphor, add the methanol and stir it with a glass rod until the camphor has dissolved. Prepare an ice bath before the next step. 2. Intermittently and in small portions, add the sodium borohydride to the dissolved camphor. If necessary, cool the reaction mixture over an ice bath to keep it at about room temperature during the addition. 3. After the reducing agent has been added, boil the solution gently for 23 minutes. 4. Allow the flask to cool for a few minutes and then carefully add ice water (approximately 3.5mL per 100mg of starting material). 5. Collect the white solid (which may be quite clumpy and wet) by vacuum filtration using a Hirsch funnel and allow it to dry somewhat with continued suction. 6. Transfer the solid to a 10mL Erienmeyer flask and dissolve it in a minimal volume of ether (about 4.0mL per 100mg of starting material). Add sodium sulfate drying agent untilit no longer clumps and the ether solution is clear. 7. Filter the mixture through a filter pipet and transfer the ether solution containing your product into a tared side-arm filtration flask that contains a boiling chip. 8. Secure the fitration flask in a warm water bath (40C5C) and evaporate the ether using an aspirator or via rotovap. When only a trace of ether remains, remove the water bath and finish the evaporation using the aspirator only. Atematively, you may use a gentle stream of nitrogen gas to complete the evaporation. 9. Determine the percent vield of your product. 10. Characterization: a. Obtain a melting point. Your meiting point will not match either of those given in the table nor will it be an average of the meiting points. Comment on why that is to be expected. b. Prepare a sample for NMR spectroscopy under your TA's supervision. A spectrum of the pure (15)() borneol starting material is attached for comparison. Based on the spectrum obtained for your reduction product, determine the disstereomeric ratio. To be addressed in your conclusion: Does the product distribution reflect what was predicted? If not, can you think of a reason whil? Can you make any of the other peak assignments? Sadtier (in the library) is a good reference for spectroscopic data of common oreanic compounds