Questions.
Record all your experimental data below, Carry out and include all the calculations needed to determine
mass and density of the liquid for both trials and for the average density of the liquid.
LAB!!

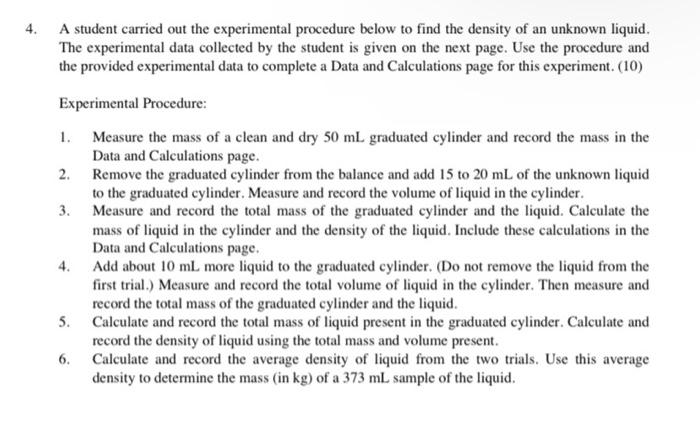
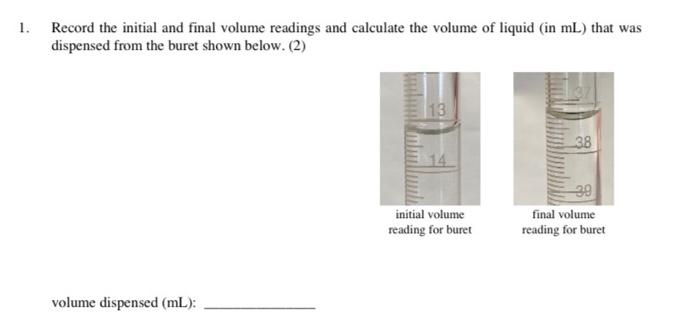
1. Record the initial and final volume readings and calculate the volume of liquid (in mL) that was dispensed from the buret shown below. (2) 13 38 14 initial volume reading for buret 39 final volume reading for buret volume dispensed (mL): 2. If 21.0 mL of a 3.00 M HNO2 solution is diluted to a volume of 1.25 L, then what is the new concentration (in M) of the HNO3 solution? How many grams of HNO3 are in this solution? (5) concentration (M): mass of HNO3 (g): 3. Find the range and average error for the set of mass measurements given below. Describe how another set of mass measurements for the sample could be more accurate than the set given below. The actual mass of the sample is 4.175 g. (4) density measurements: 4.715 g 4.721 g 4.714 g 4.726 g range: average error: 4. A student carried out the experimental procedure below to find the density of an unknown liquid. The experimental data collected by the student is given on the next page. Use the procedure and the provided experimental data to complete a Data and Calculations page for this experiment. (10) Experimental Procedure: 1. Measure the mass of a clean and dry 50 mL graduated cylinder and record the mass in the Data and Calculations page. 2. Remove the graduated cylinder from the balance and add 15 to 20 mL of the unknown liquid to the graduated cylinder. Measure and record the volume of liquid in the cylinder 3. Measure and record the total mass of the graduated cylinder and the liquid. Calculate the mass of liquid in the cylinder and the density of the liquid. Include these calculations in the Data and Calculations page. 4. Add about 10 mL more liquid to the graduated cylinder. (Do not remove the liquid from the first trial.) Measure and record the total volume of liquid in the cylinder. Then measure and record the total mass of the graduated cylinder and the liquid. 5. Calculate and record the total mass of liquid present in the graduated cylinder. Calculate and record the density of liquid using the total mass and volume present. 6. Calculate and record the average density of liquid from the two trials. Use this average density to determine the mass (in kg) of a 373 mL sample of the liquid. Experimental Data for Measuring the Density of the Unknown Liquid Trial 1 Data (Steps 1-3): 20 HEIGH 38939, 10 liquid volume mass of the cylinder mass of the cylinder and liquid Trial 2 Data (Steps 4-5): 30 20 49028 liquid volume mass of the cylinder and liquid