Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Questions to Address in Your Report 1) From the spectral data ( 1 H, IR, MS) you were given, identify the structure of your product
Questions to Address in Your Report
1) From the spectral data (1H, IR, MS) you were given, identify the structure of your product. Explain why you chose your particular product based on the spectroscopic data. You will not receive full marks for determination of the unknown unless you explain why.
2) Would you suggest that your purified compound is greater than 95% pure based on the IR spectrum and melting point?
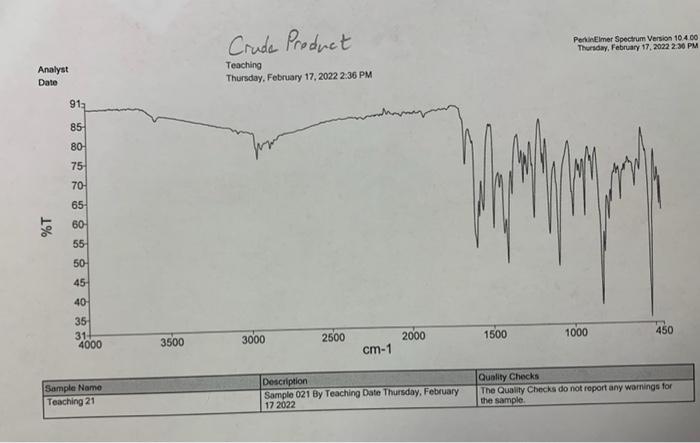
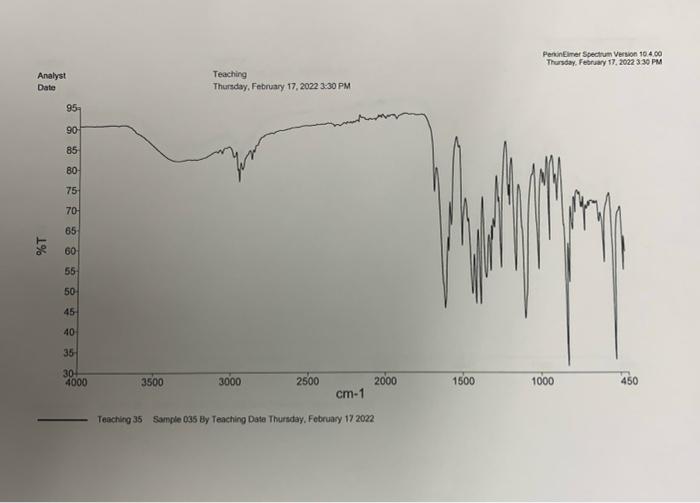
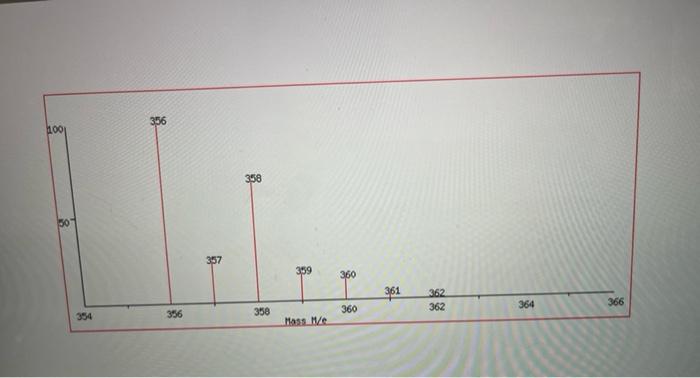

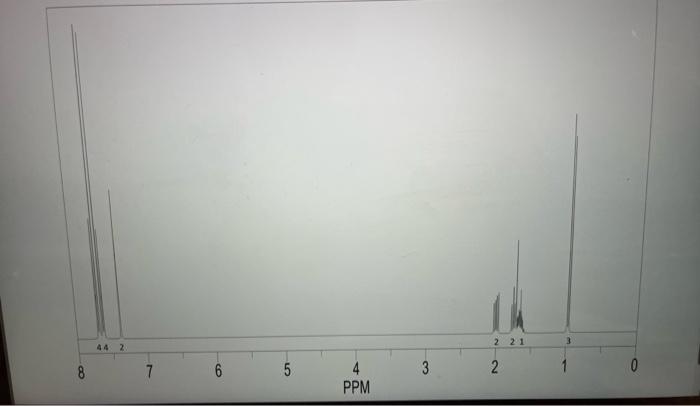
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


