Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(Quick Bro) A) Determine whether the following reactions are redox reaction, not a redox reaction or disproportionation reaction. You are required to show the oxidation
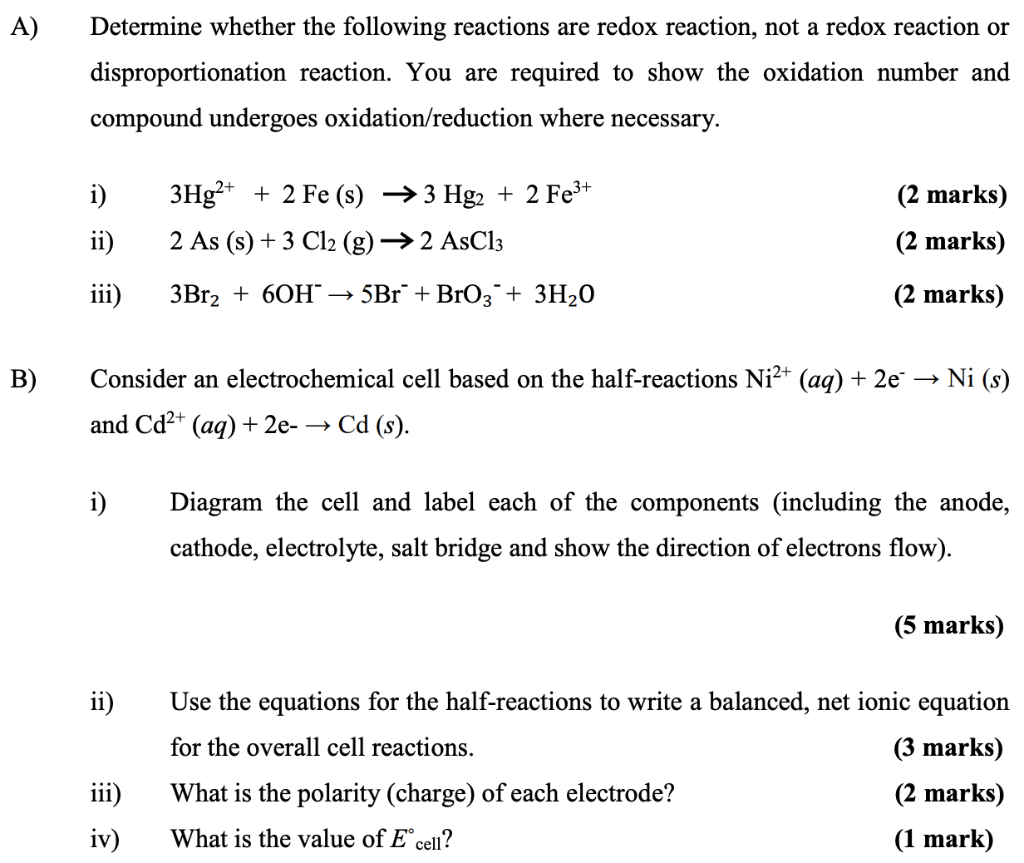
(Quick Bro)
A) Determine whether the following reactions are redox reaction, not a redox reaction or disproportionation reaction. You are required to show the oxidation number and compound undergoes oxidation/reduction where necessary. i) (2 marks) 3Hg2+ + 2 Fe (s) + 3 Hg2 + 2 Fe3+ 2 As (s) + 3 Cl2 (g) 2 AsCl3 ii) (2 marks) iii) 3Br2 + 60H 5Br + BrO3 + 3H2O (2 marks) B) - Consider an electrochemical cell based on the half-reactions Ni2+ (aq) + 2e Ni (s) and Cd2+ (aq) + 2e- Cd (s). i) Diagram the cell and label each of the components (including the anode, cathode, electrolyte, salt bridge and show the direction of electrons flow). (5 marks) ii) Use the equations for the half-reactions to write a balanced, net ionic equation for the overall cell reactions. (3 marks) What is the polarity (charge) of each electrode? (2 marks) What is the value of Ecell? (1 mark) iii) iv)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


