Answered step by step
Verified Expert Solution
Question
1 Approved Answer
31,405 Quiz: Exam 2 1. Consider the two molecules below. Propanoic acid 2 3 H H H 2 HO: O H -c-c-c-: -C- H
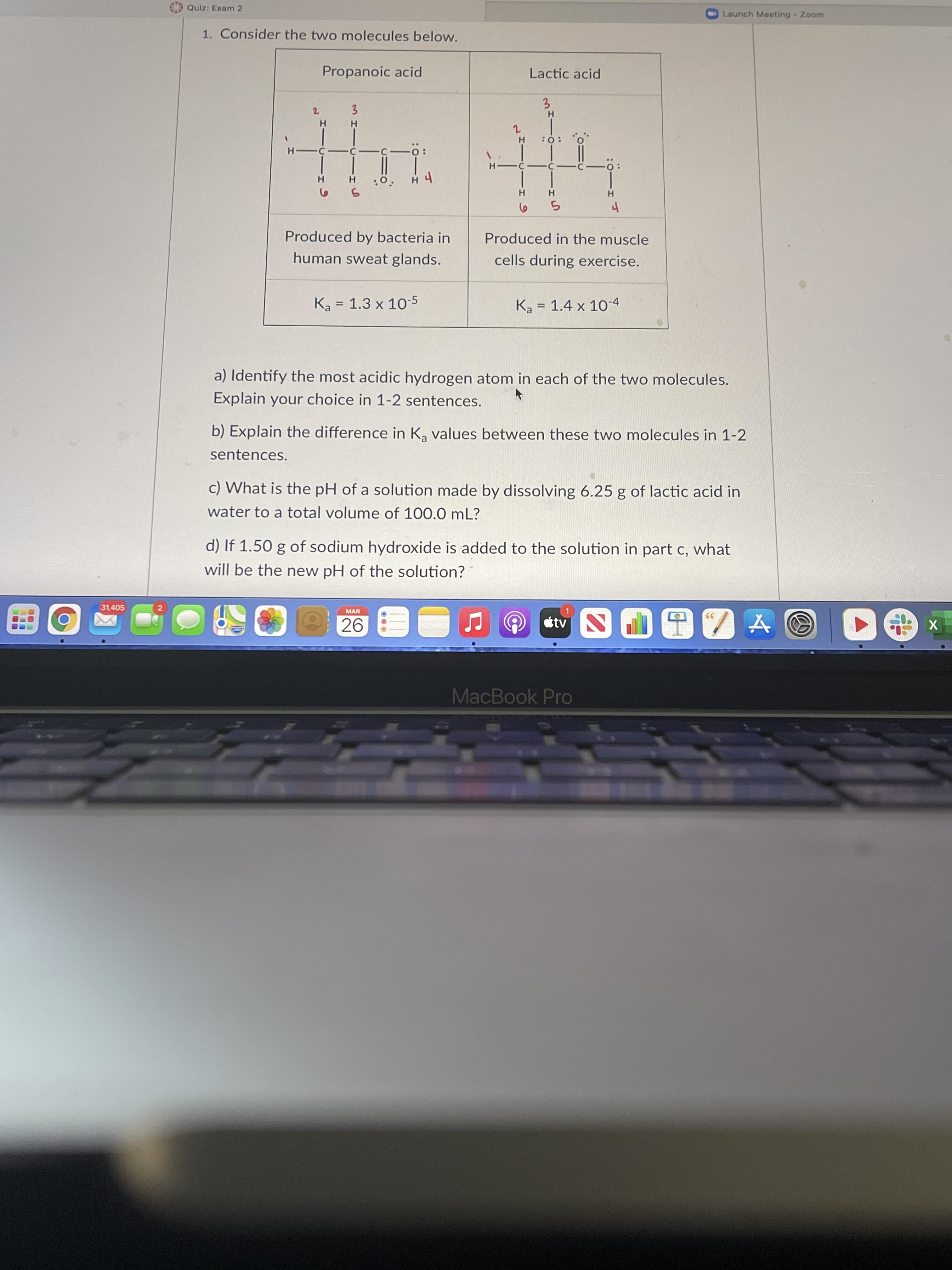
31,405 Quiz: Exam 2 1. Consider the two molecules below. Propanoic acid 2 3 H H H 2 HO: O H -c-c-c-: -C- H :0. H 4 5 6 5 4 Produced by bacteria in human sweat glands. Produced in the muscle cells during exercise. Ka 1.3 x 10-5 = Ka 1.4 x 10-4 = a) Identify the most acidic hydrogen atom in each of the two molecules. Explain your choice in 1-2 sentences. b) Explain the difference in K values between these two molecules in 1-2 sentences. c) What is the pH of a solution made by dissolving 6.25 g of lactic acid in water to a total volume of 100.0 mL? d) If 1.50 g of sodium hydroxide is added to the solution in part c, what will be the new pH of the solution? 2 MAR 26 tv DIZA MacBook Pro H- Lactic acid 3 -: H Launch Meeting - Zoom X
Step by Step Solution
★★★★★
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
1a The proton attached with the carboxylic acid group is the most acidic one because of the equivale...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


