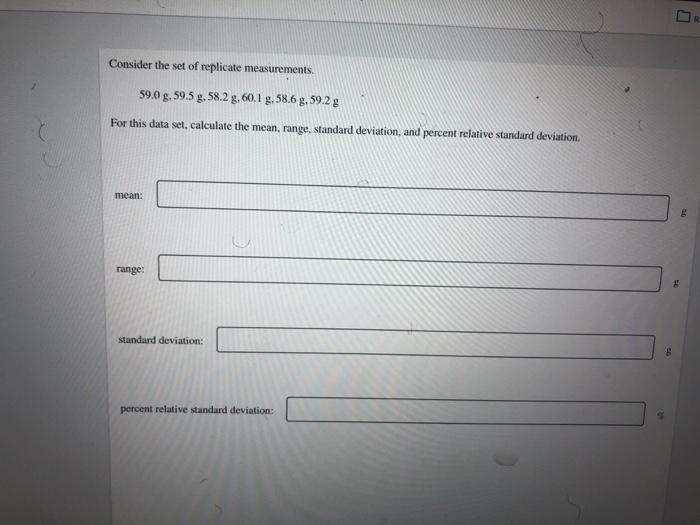
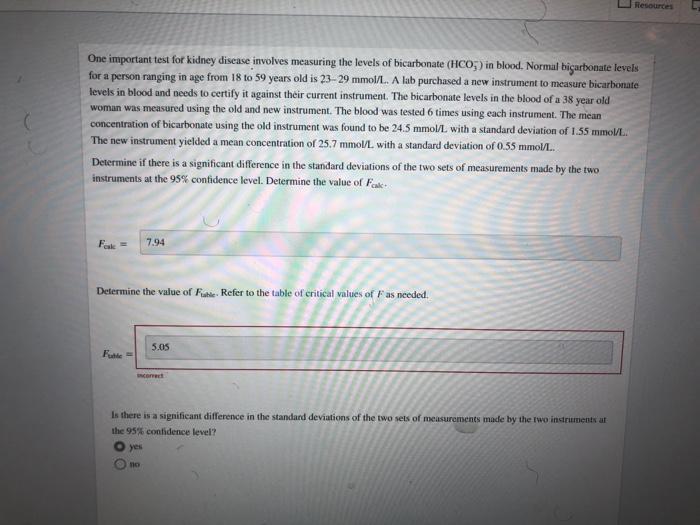
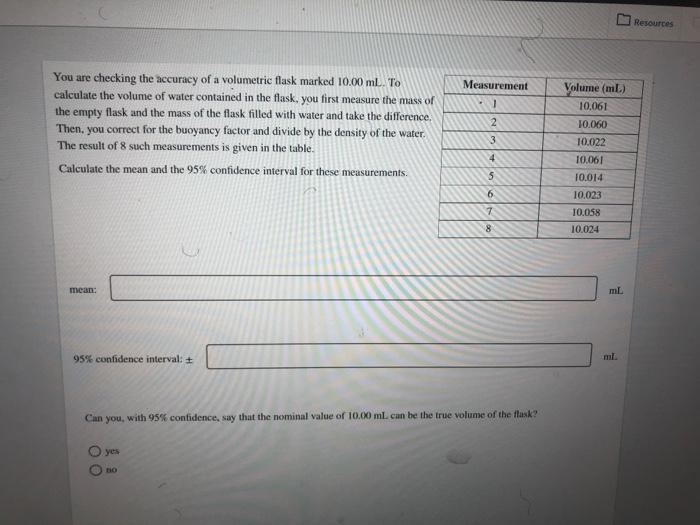
R Consider the set of replicate measurements. 59.0 g. 59.5 g. 58.2 9.60.1 g, 58.6 g. 59.2 g For this data set, calculate the mean, range. standard deviation, and percent relative standard deviation mean: range: standard deviation: percent relative standard deviation: Resouro One important test for kidney disease involves measuring the levels of bicarbonate (HCO3) in blood. Normal bicarbonate levels for a person ranging in age from 18 to 59 years old is 23-29 mmol/L. A lab purchased a new instrument to measure bicarbonate levels in blood and needs to certify it against their current instrument. The bicarbonate levels in the blood of a 38 year old woman was measured using the old and new instrument. The blood was tested 6 times using each instrument. The mean concentration of bicarbonate using the old instrument was found to be 24.5 mmol/L with a standard deviation of 1.55 mmol/L The new instrument yielded a mean concentration of 25.7 mmol/l with a standard deviation of 0.55 mmol/L. Determine if there is a significant difference in the standard deviations of the two sets of measurements made by the two instruments at the 95% confidence level. Determine the value of Fake Fok 7.94 Determine the value of Fable. Refer to the table of critical values of Fas needed. 5.05 Fuatie Is there is a significant difference in the standard deviations of the two sets of measurements made by the two instruments ar the 95% confidence level? yes 10 Resources Measurement You are checking the accuracy of a volumetric flask marked 10.00 ml. To calculate the volume of water contained in the flask. you first measure the mass of the empty flask and the mass of the flask filled with water and take the difference. Then, you correct for the buoyancy factor and divide by the density of the water. The result of 8 such measurements is given in the table. Calculate the mean and the 95% confidence interval for these measurements. -] 2 3 4 Volume (mL) 10.061 10.060 10.022 10.061 10.014 10.023 10.058 10.024 5 6 7 8 mean: mL 95% confidence intervals + Can you, with 95% contidence, say that the nominal value of 10.00 ml. can be the true volume of the task? yes DO