Question
Rank the volatility of the following in water from lowest to highest. CH3 CH,-C-CH, CH; neopentane 2-methylbutan-2-ol 2,3-dimethylbutane pentan-1-ol hexane 9. Which intermolecular forces
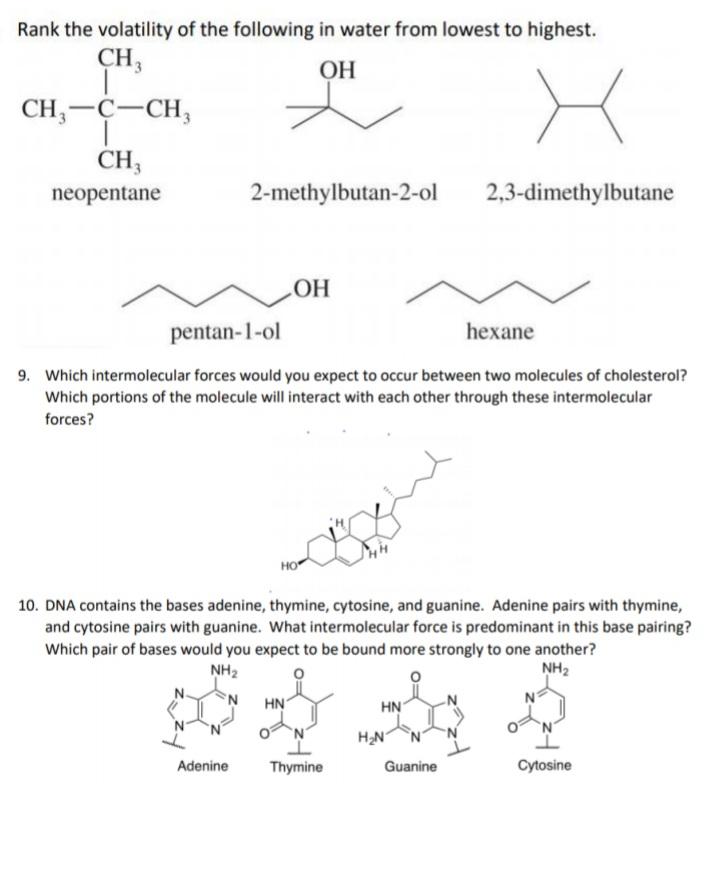
Rank the volatility of the following in water from lowest to highest. CH3 CH,-C-CH, CH; neopentane 2-methylbutan-2-ol 2,3-dimethylbutane pentan-1-ol hexane 9. Which intermolecular forces would you expect to occur between two molecules of cholesterol? Which portions of the molecule will interact with each other through these intermolecular forces? 10. DNA contains the bases adenine, thymine, cytosine, and guanine. Adenine pairs with thymine, and cytosine pairs with guanine. What intermolecular force is predominant in this base pairing? Which pair of bases would you expect to be bound more strongly to one another? NH2 NH2 'N. HN HN H2N N Adenine Thymine Guanine Cytosine
Step by Step Solution
3.56 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Bank Management
Authors: Timothy W. Koch, S. Scott MacDonald
8th edition
1133494684, 978-1305177239, 1305177231, 978-1133494683
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



