Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Rate Law for Two Reactant Systems For the reaction A +B +C the rate law can be written as: Rate = k[A]m[B] where k
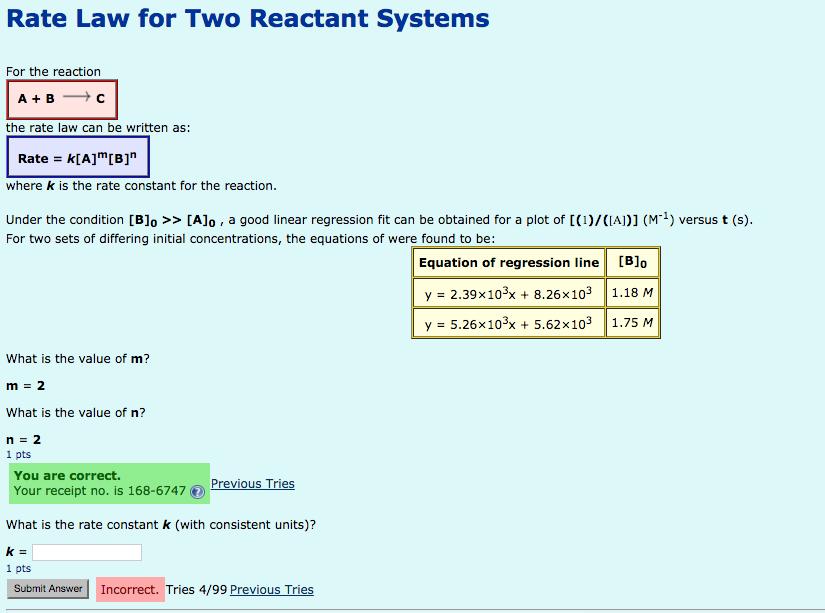
Rate Law for Two Reactant Systems For the reaction A +B +C the rate law can be written as: Rate = k[A]m[B]" where k is the rate constant for the reaction. Under the condition [B]o >> [A]o , a good linear regression fit can be obtained for a plot of [(1)/(IA)] (M) versus t (s). For two sets of differing initial concentrations, the equations of were found to be: Equation of regression line [B]o y = 2.39x103x + 8.26x103 1.18 M y = 5.26x103x + 5.62x103 1.75 M What is the value of m? m = 2 What is the value of n? n = 2 1 pts You are correct. Your receipt no. is 168-6747 Previous Tries What is the rate constant k (with consistent units)? k = 1 pts Incorrect. Tries 4/99 Previous Tries Submit Answer
Step by Step Solution
★★★★★
3.45 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
rate KAM Bn when BoAo the rate becomes r K Aom where K KBom K is the rate constant m is ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


