Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Rate of Leaching. In studying the rate of leaching of a substance A from solid particles by a solvent B, we may postulate that the
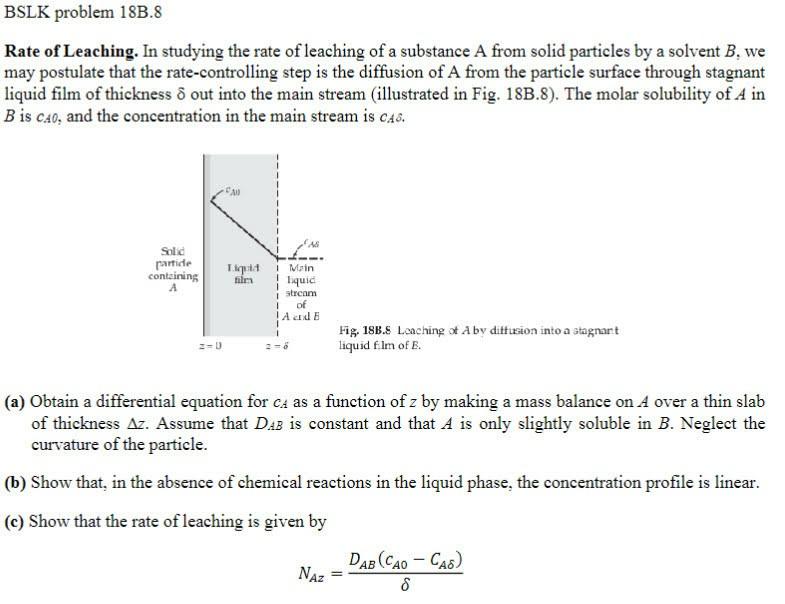
Rate of Leaching. In studying the rate of leaching of a substance A from solid particles by a solvent B, we may postulate that the rate-controlling step is the diffusion of A from the particle surface through stagnant liquid film of thickness out into the main stream (illustrated in Fig. 18B.8). The molar solubility of A in B is CA0, and the concentration in the main stream is CA. Fig. 18B. S Laching of A by dittusion into a atagnant iquid flm of E. (a) Obtain a differential equation for cA as a function of z by making a mass balance on A over a thin slab of thickness z. Assume that DAB is constant and that A is only slightly soluble in B. Neglect the curvature of the particle. (b) Show that, in the absence of chemical reactions in the liquid phase, the concentration profile is linear. (c) Show that the rate of leaching is given by NAz=DAB(cA0CA)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


