Question
Reaction is the first order in [A]. Consider the following data: Time [A] M 0.0 1.60 10.0 0.40 20.0 0.10 The rate constant for this
Reaction 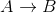 is the first order in [A]. Consider the following data:
is the first order in [A]. Consider the following data:
| Time | [A] M |
| 0.0 | 1.60 |
| 10.0 | 0.40 |
| 20.0 | 0.10 |
The rate constant for this experiment is ____
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Hny cuer A B Time CAI M O o 100 200 160 040 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



