Answered step by step
Verified Expert Solution
Question
1 Approved Answer
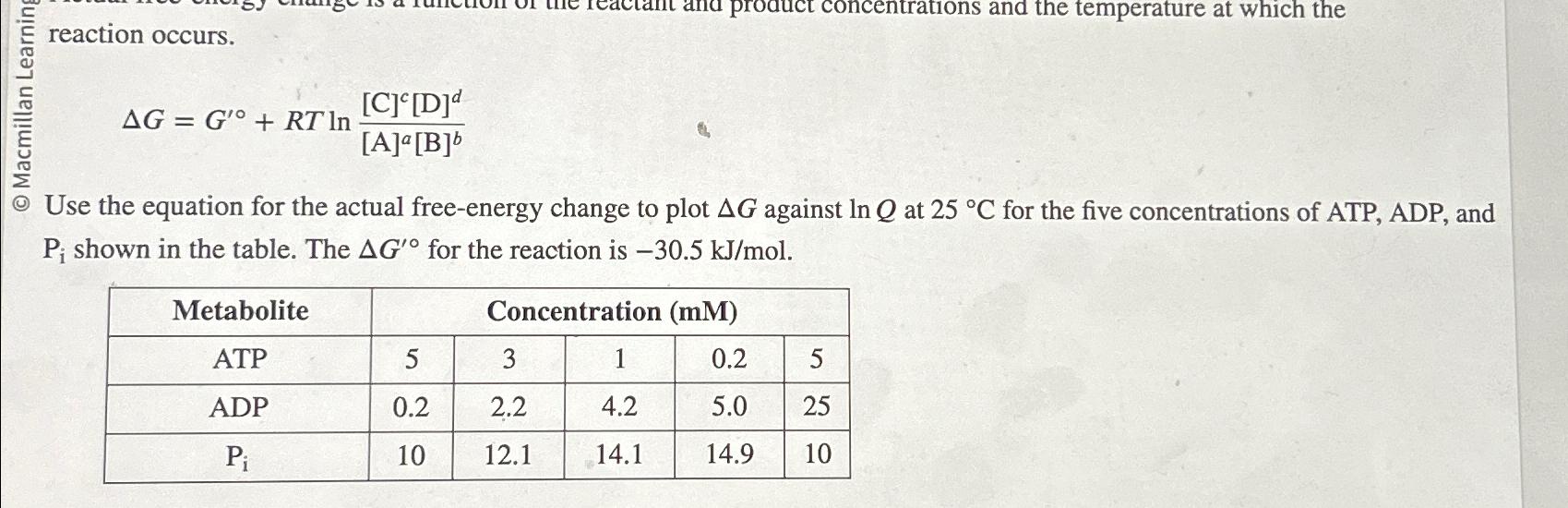
reaction occurs. Delta G=G^('@)+RTln([C]^(c)[D]^(d))/([(A)]^(a)[(B)]^(b)) Use the equation for the actual free-energy change to plot Delta G against lnQ at 25deg C for the five
reaction occurs.\
\\\\Delta G=G^('@)+RTln([C]^(c)[D]^(d))/([(A)]^(a)[(B)]^(b))\ Use the equation for the actual free-energy change to plot
\\\\Delta Gagainst
lnQat
25\\\\deg Cfor the five concentrations of ATP, ADP, and
P_(i)shown in the table. The
\\\\Delta G^('@)for the reaction is
-30.5k(J)/(m)ol.\ \\\\table[[Metabolite,Concentration (mM)],[ATP,5,3,1,0.2,5],[ADP,0.2,2.2,4.2,5.0,25],[
P_(i),10,12.1,14.1,14.9,10]]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


