Answered step by step
Verified Expert Solution
Question
1 Approved Answer
reactor design Question 3 Consider the following gas-phase reaction is carried out isothermally first in a flow reactor. 3 N+H, NH, 2 The molar feed
reactor design 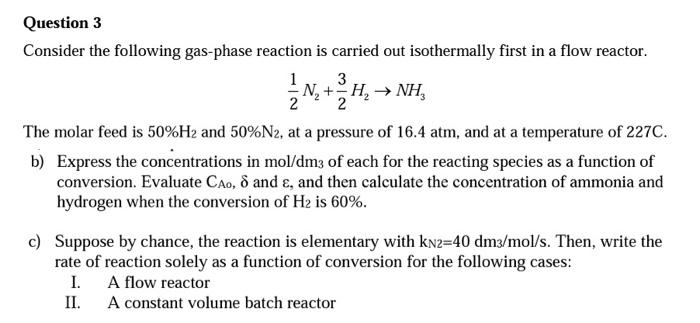
Question 3 Consider the following gas-phase reaction is carried out isothermally first in a flow reactor. 3 N+H, NH, 2 The molar feed is 50%H2 and 50%N2, at a pressure of 16.4 atm, and at a temperature of 227C. b) Express the concentrations in mol/dm3 of each for the reacting species as a function of conversion. Evaluate Cao, d and , and then calculate the concentration of ammonia and hydrogen when the conversion of H2 is 60%. c) Suppose by chance, the reaction is elementary with kn2=40 dm3/mol/s. Then, write the rate of reaction solely as a function of conversion for the following cases: I. A flow reactor II. A constant volume batch reactor 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


