Answered step by step
Verified Expert Solution
Question
1 Approved Answer
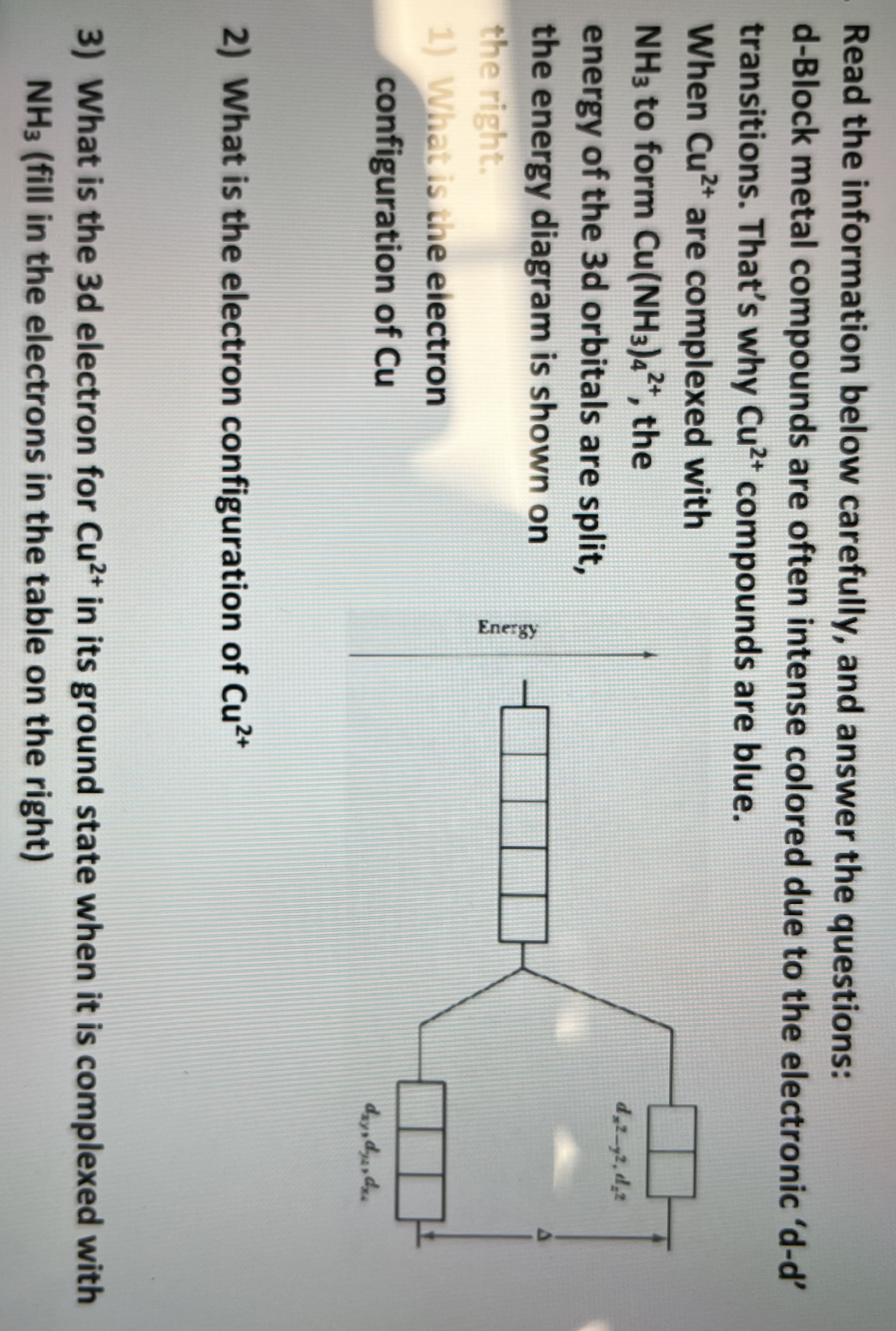
Read the information below carefully, and answer the questions: d - Block metal compounds are often intense colored due to the electronic ' d -
Read the information below carefully, and answer the questions:
dBlock metal compounds are often intense colored due to the electronic
transitions. That's why compounds are blue.
When are complexed with
to form the
energy of the orbitals are split,
the energy diagram is shown on
the right.
What is the eiectron
configuration of
What is the electron configuration of
What is the d electron for in its ground state when it is complexed with
fill in the electrons in the table on the right
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


