Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Real world importance of IM forces: Did you know that hydrogen bonding is critical to the operation of DNA? Hydrogen bonds hold together the two
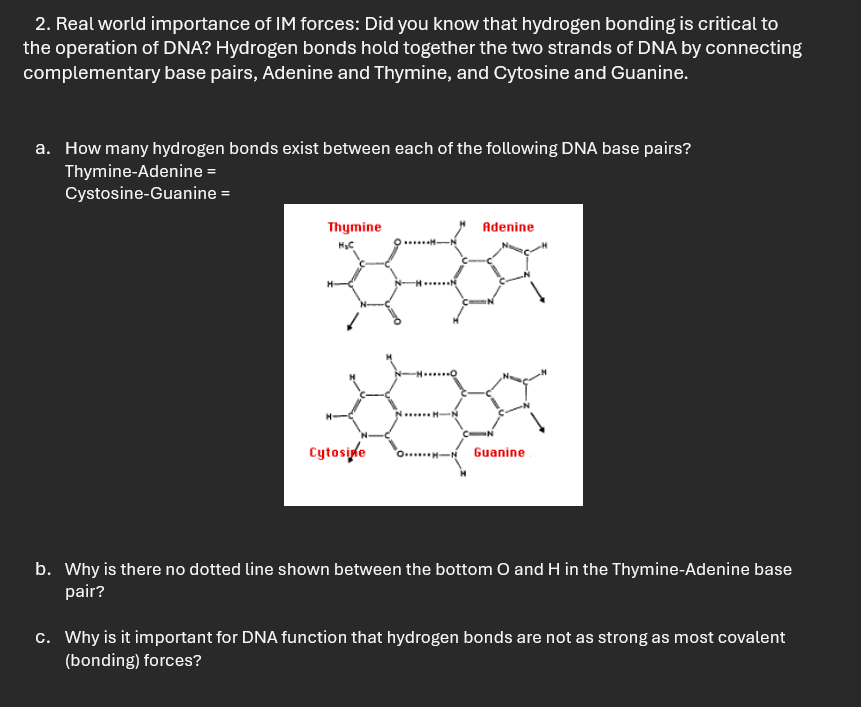
Real world importance of IM forces: Did you know that hydrogen bonding is critical to the operation of DNA? Hydrogen bonds hold together the two strands of DNA by connecting complementary base pairs, Adenine and Thymine, and Cytosine and Guanine.
a How many hydrogen bonds exist between each of the following DNA base pairs?
ThymineAdenine
CystosineGuanine
b Why is there no dotted line shown between the bottom and in the ThymineAdenine base pair?
c Why is it important for DNA function that hydrogen bonds are not as strong as most covalent bonding forces?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


