Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Reamer, Sage and Lacey [Ind. Eng. Chem., 43, 1436 (1951)] measured the following compositions of phases in equilibrium for the methane-butane-decane system at 280F and
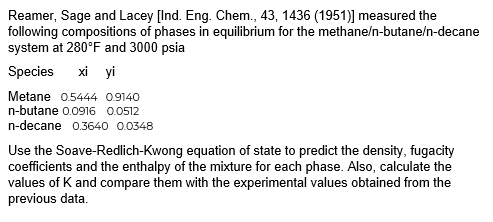
Reamer, Sage and Lacey [Ind. Eng. Chem., 43, 1436 (1951)] measured the following compositions of phases in equilibrium for the methane-butane-decane system at 280F and 3000 psia
Reamer, Sage and Lacey [Ind. Eng. Chem., 43, 1436 (1951)] measured the following compositions of phases in equilibrium for the methane-butane-decane system at 280F and 3000 psia Use the Soave-Redlich-Kwong equation of state to predict the density, fugacity coefficients and the enthalpy of the mixture for each phase. Also, calculate the values of K and compare them with the experimental values obtained from the previous dataStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


