Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Refer to the CRC Handbook, Lange's Handbook, or the Aldrich Handbook to find the boiling point, index of refraction Pip, and density for each compound
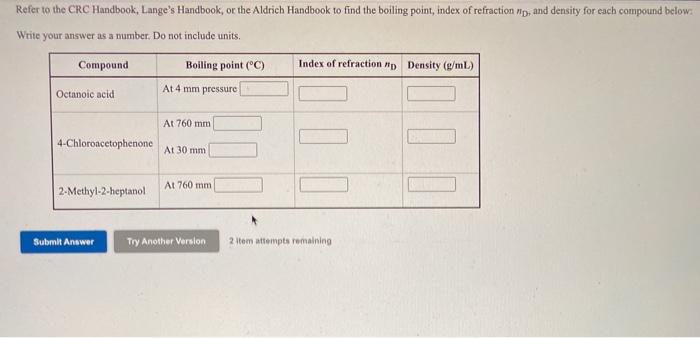
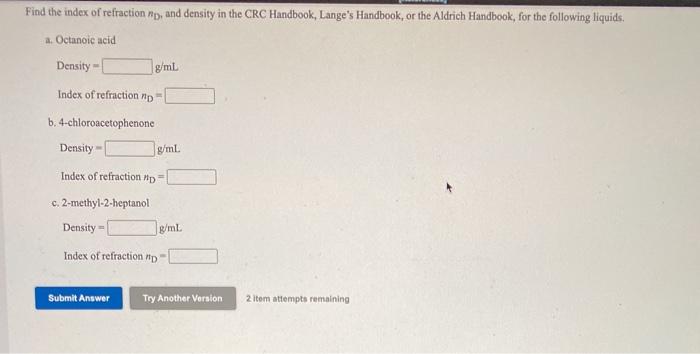
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


