Refer to the temperature versus time graph (Figure 2) when answering the questions in Parts C through F. A system consists of 250 g of
Refer to the temperature versus time graph (Figure 2) when answering the questions in Parts C through F. A system consists of 250 g of water. The system, originally at T A = 21.0 ?C, is placed in a freezer, where energy is removed from it in the form of heat at a constant rate. The figure shows how the temperature of the system takes t 1 = 10 min = 600 s to drop to 0? C, after which the water freezes. Once the freezing is complete, the temperature of the resulting ice continues to drop, reaching temperature T B after an hour. The following specific heat and latent heat values for water may be helpful.
How much energy must be transferred out of the system as heat Q to lower its temperature to 0?C?
ANSWER Q = 2.20 x 10^4
Cooling Power of P is 36.6 W
At what time t 2 will the water be completely frozen so the temperature can begin to fall below 0?C?
(seconds)
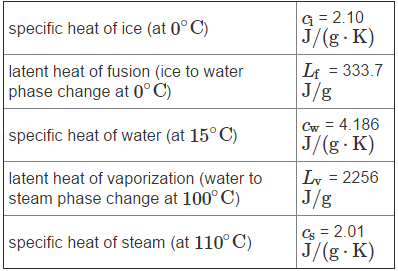
specific heat of ice (at 0C) latent heat of fusion (ice to water phase change at 0C) specific heat of water (at 15C) latent heat of vaporization (water to steam phase change at 100 C) specific heat of steam (at 110 C) G = 2.10 J/(g.K) Lf = 333.7 J/g Cw = 4.186 J/(g.K) Lv = 2256 J/g Cs = 2.01 J/(g.K)
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
Mass of water m 025 kg Temperature TA 21C TB ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


