Reference the Excel file containing financial information from footnote disclosures and substantive analytical procedures using data analytics from the Module 8 folder.
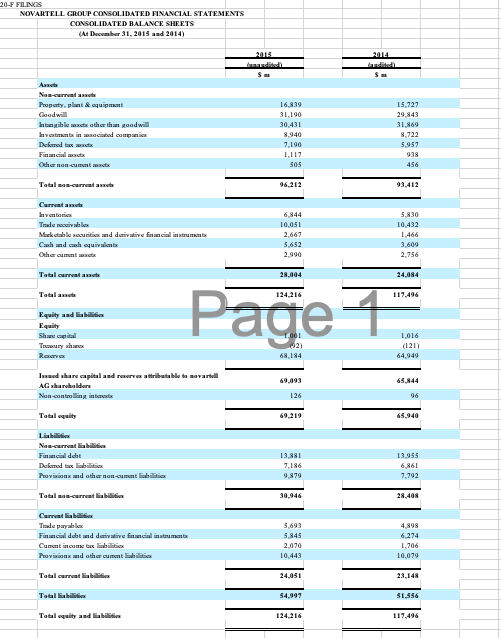
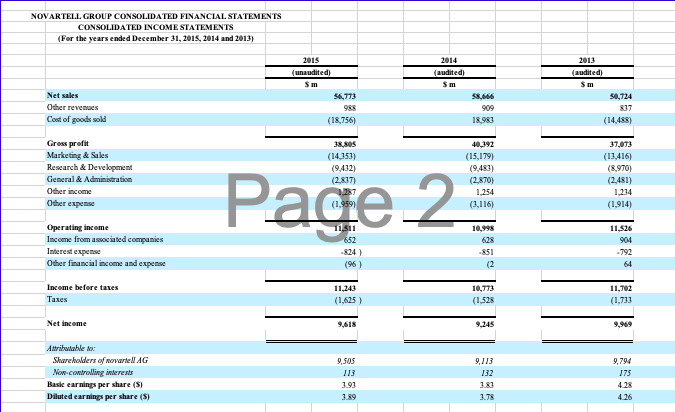
PharmaCorp will be used as the main analytical procedure tasks you will want to focus on for this assignment. The other companies, Novartell and AstraZoro, will be used as industry comparisons.
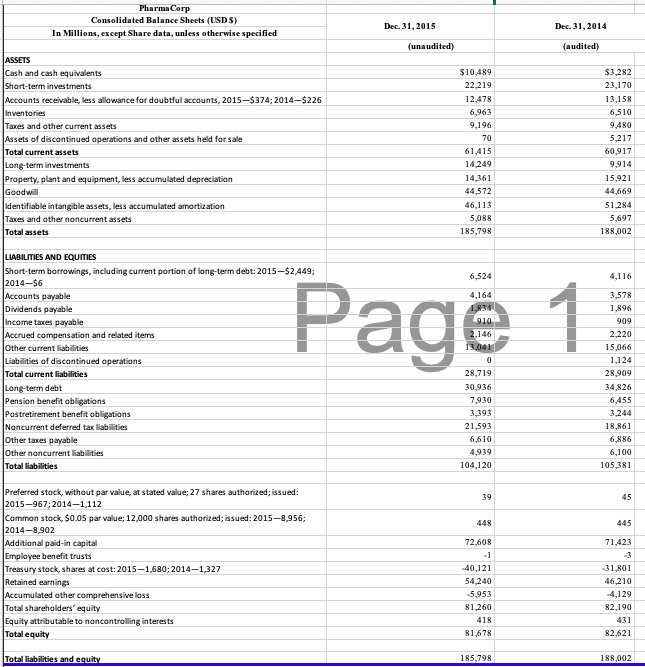
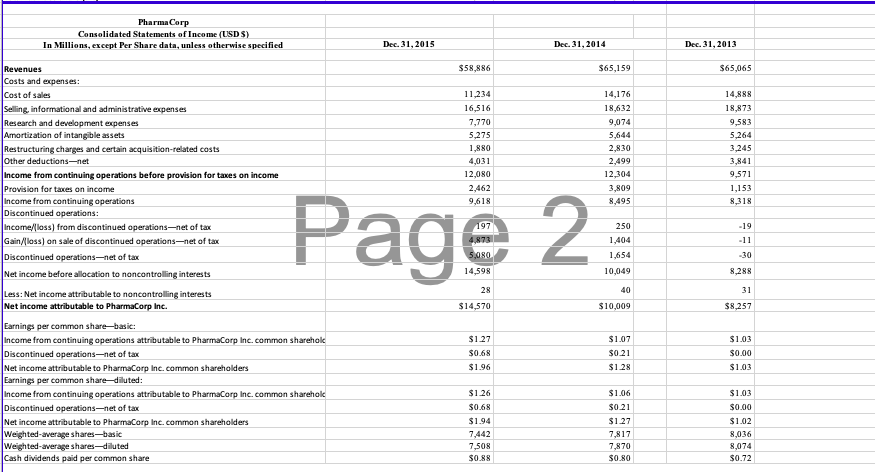
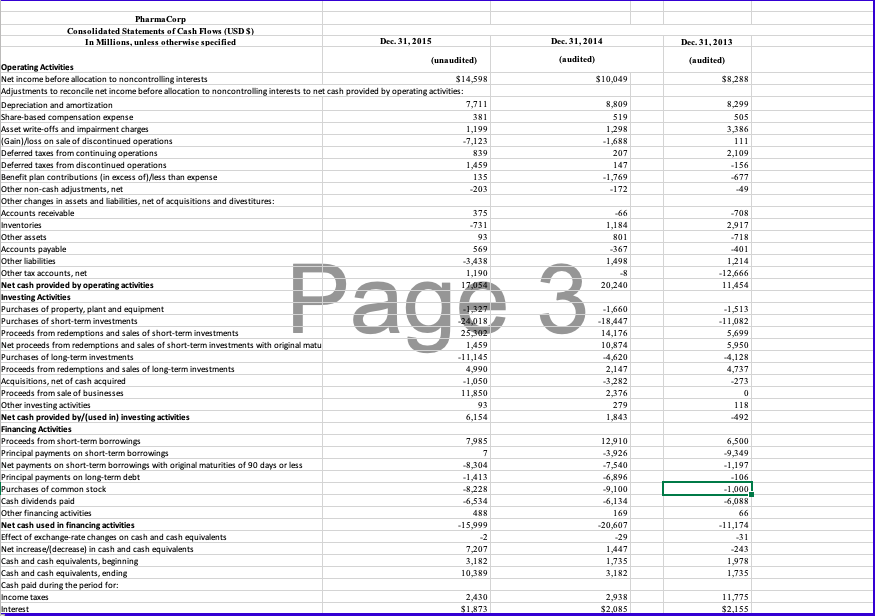
The opportunity exists in this case to perform planning and substantive analytical procedures for accounts in the revenue cycle. You may assume that the 2015 financial information is unaudited, but the information from 2014 has been audited. Consider the following trends and characteristics of the pharmaceutical industry and for PharmaCorp in particular as you work on this case:
- Following many years of dominant financial performance by companies in the United States, Europe and Canada, increased competition is arising from organizations in emerging economies such as Brazil, India, and China.
- Significant uncertainty exists in the industry due to regulation covering health-care and government reimbursements related to certain procedures and prescribed pharmaceuticals.
- Policy makers in the industry and governments increasingly:
- Mandate necessary prescripts for patients
- Focus on prevention instead of treatment regimes, thereby leading to changes in demand for some products
- Anticipated growth in the industry is expected to be 5% to 7% in 2016 compared with 4% to 5% in the prior year as stated by leading industry analysts.
- Pharmacorp started and executed a significant cost reduction initiative aimed at improving efficiency, reducing research and development costs, and eliminating corporate overhead in 2014.
- PharmaCorps credit policies has remained the same over the past several years. Their credit policies are considered stringent in their industry, and they have been criticized on occasion for these policies in relation to their competitors.
- Two of the companies most popular pharmaceuticals, Sistosis and Vigarvox, are no longer patented as of the last quarter of 2015 and are now facing competition from generic alternatives.
Required:
Part I: Planning Analytical Procedures
- Step 1: Identify Proper Analytical Procedures. The senior auditor suggests you should use these ratios (on the financial statement level) for planning the analytical procedures as part of the revenue cycle at the company:
- Gross margin: (revenues-cost of sales)/revenues
- Turnover of receivables: (revenues/average accounts receivable); use the ending accounts receivable
- Receivables as a percentage of current assets: (accounts receivable/total current assets)
- Receivables as a percentage of total assets: (accounts receivable/total assets)
- Allowance for uncollectible accounts as a percentage of accounts receivable: (allowance/accounts receivable)
- Identify other relationships or trends that are relevant as part of the planning analytics. Discuss your reasons for your choices.
- Step 2: Evaluate the Data Reliability When Developing Expectations. The data you will use to develop expectations in the revenue cycle has been deemed reliable by the audit staff.
- Discuss the likely factors the audit team will consider when making this determination.
- Step 3: Develop expectations for accounts in the revenue cycle and for the ratios from Step # 1 that you deem as relevant. Since this is a planning analytical procedure, the expectations are not set at a high a high level of precision. Indicate if you expect a ratio to rise, fall, or remain the same, and explain the level of any anticipated rises or falls, or the range of the ratio. Pharma Corps financial information is in the first tab of the Excel worksheet, while the information for Novartell and AstraZoro is available in the last two tabs of the file.
- Consider both historical trends of Pharmcorp and the industry on the whole.
- Step 4 and Step 5: Define and Identify Substantial Unanticipated Variances. Refer to the text for guidance on materiality.
- Apply those guidelines to Step 4 of planning the analytical procedures as part of the revenue cycle for Pharmacorp. Define the meaning of a significant difference. Discuss your reasons for these choices. Discuss the qualitative materiality considerations in relation to this case.
- Once you have determined the levels of difference you would consider noteworthy, calculate the Step 1 ratios (and any additional trend or ration analysis you deemed necessary), based on Pharmacorps financial statement figures. Identify the ratios where you expect a significant difference.
- Step 6 and Step 7: Investigate Substantial Unanticipated Variances and Ensure Appropriate Documentation.
- Discuss the accounts or relationships you feel should be investigated further using substantive audit procedures. Discuss your reasons for these choices.
- Describe the information that should be a part of the auditors report or files.
Part II: Substantive Analytical Procedures
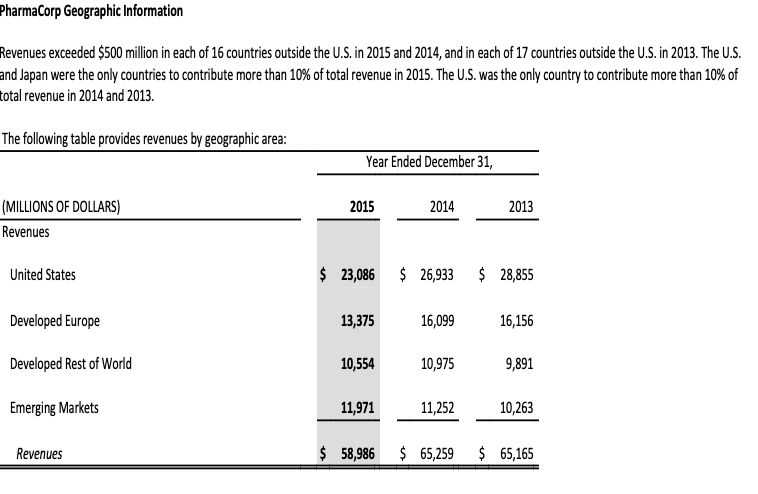
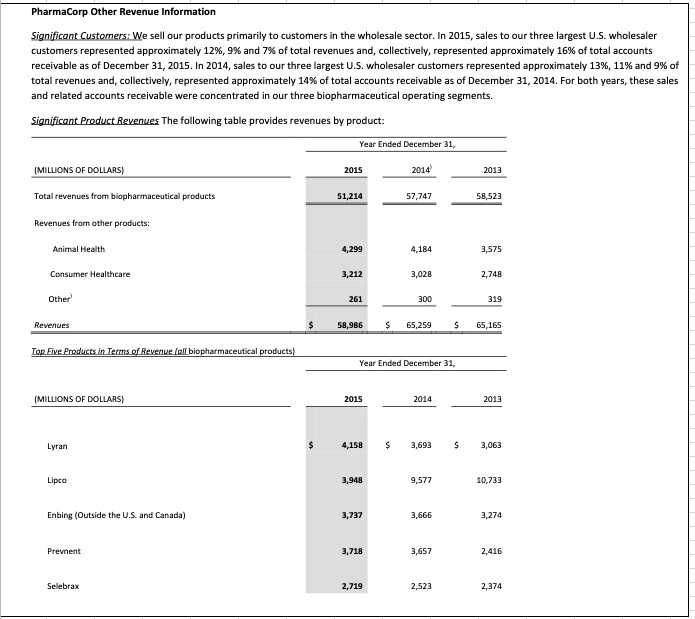
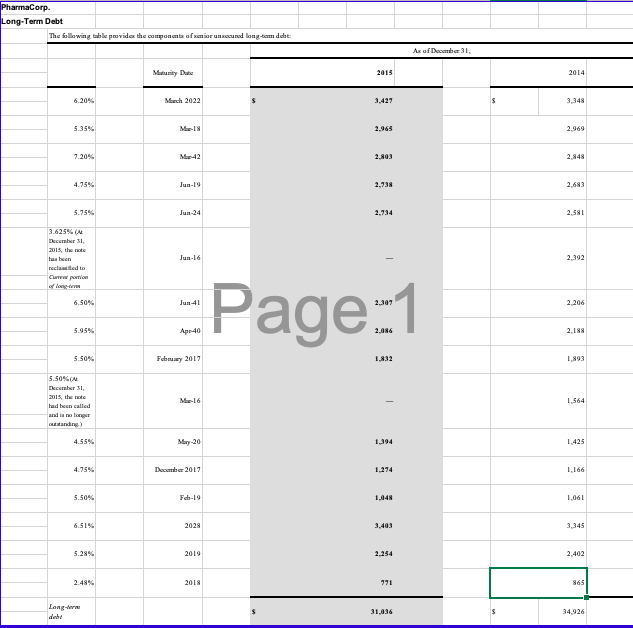
- You will see three tabs in the Excel file that should be reviewed: the Pharmacorp Segment Information, Pharmacorps Geographic Information, and Pharmacorps Other Revenue Information. These tabs display excerpts from Pharma Corps footnote disclosures regarding segment, geographic, and other revenue information. Examine these disclosures and discuss the operating segments and geographic regions where the company does business.
- Which operating segments generate the most revenue for the company and may be considered the most important? Which regions are the most important to the organization from geographic standpoint? List the three most important products manufactured by Pharmacorp? Discuss any trends you notice in relation to revenue generation for each of these different categories.
- Explain the different types of ratio analysis that could be conducted in substantive analytical procedures using the data from the segment, geographic, and other revenue information. An example would be the R&D expenses as a percentage of revenues. How would these substantive analytics be different from the planning analytics? Discuss the trends and relationships that are relevant, and what are the implications in relation to further substantive testing?

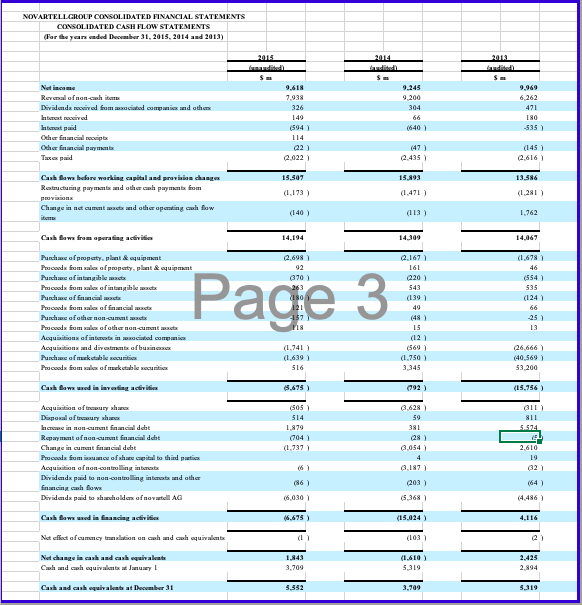
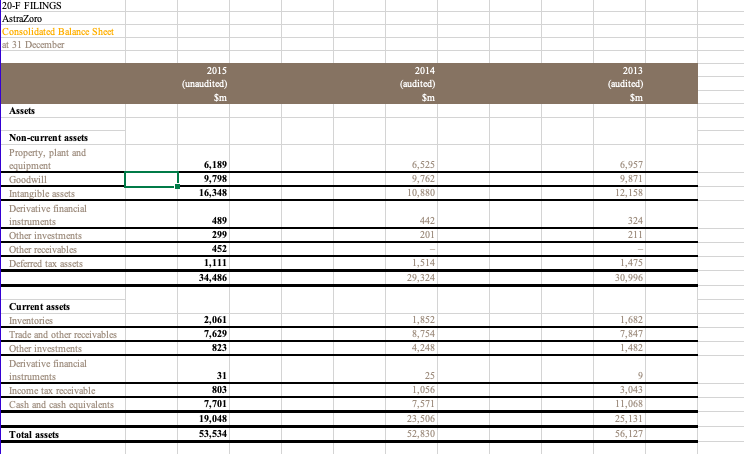

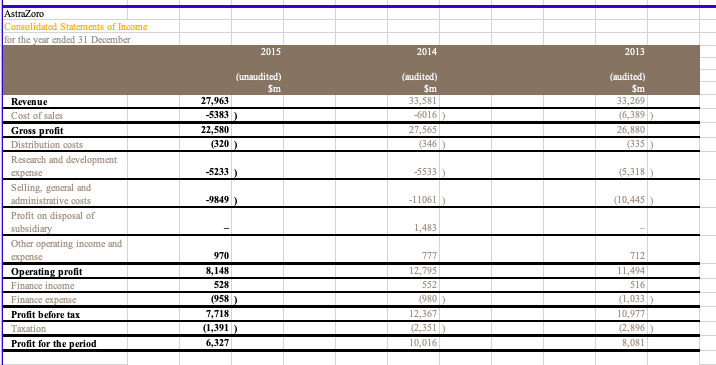

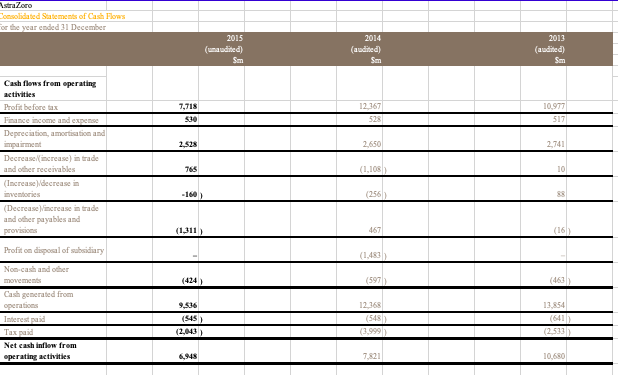

Pharma Corp Consolidated Balance Sheets (USDS) In Millions, except Share data, unless otherwise specified Dec 31, 2015 Dec 31, 2014 (unaudited) (audited) ASSETS Cash and cash equivalents Short-term investments Accounts receivable, less allowance for doubtful accounts, 2015-$374;2014-$226 Inventories Taxes and other current assets Assets of discontinued operations and other assets held for sale Total current assets Long-term investments Property, plant and equipment, less accumulated depreciation Goodwill Identifiable intangible assets, less accumulated amortization Taxes and other noncurrent assets Total assets $10,489 22,219 12,478 6,963 9,196 70 61,415 14.249 14,361 44,572 46,113 5.088 185,798 $3,282 23,170 13,158 6,510 9,480 5.217 60,917 9,914 15,921 44,669 51,284 5,697 188 6,524 4,116 4,164 LIABILITIES AND EQUITIES Short-term borrowings, including current portion of long-term debt: 2015-$2,449; 2014-$6 Accounts payable Dividends payable Income taxes payable Accrued compensation and related items Other current liabilities Liabilities of discontinued operations Total current liabilities Long-term debt Pension benefit obligations Postretirement benefit obligations Noncurrent deferred tax liabilities Other taxes payable Other noncurrent liabilities Total liabilities 910 2.146 13.041 0 28,719 30,936 7,930 3,393 21,593 6,610 4,939 104,120 3,578 1,896 909 2,220 15,066 1,124 28,909 34,826 6,455 3,244 18,861 6,886 6,100 105,381 39 45 445 Preferred stack, without par value, at stated value: 27 shares authorized; issued: 2015967,2014-1,112 Common stock, $0.05 par value: 12,000 shares authorized; issued: 2015-8,956; 2014-8,902 Additional paid-in capital Employee benefit trusts Treasury stack, shares at cost: 2015-1,680; 2014-1,327 Retained earnings Accumulated other comprehensive loss Total shareholders' equity Equity attributable to noncontrolling interests Total equity 72,608 -1 -40,121 54,240 -5,953 81,260 418 81,678 71,423 -3 -31,801 46,210 -4,129 82,190 431 82.621 Total liabilities and equity 185,798 188,002 PharmaCorp Consolidated Statements of Income (USDS) In Millions, except Per Share data, unless otherwise specified Dec 31, 2015 Dec 31, 2014 Dec. 31, 2013 $58,886 $65,159 $65,065 11,234 16,516 7,770 5,275 1,880 4,031 12,080 2.462 9,618 14,176 18,632 9,074 5,644 2,830 2,499 12,304 3,809 8,495 14,888 18,873 9,583 5,264 3,245 3,841 9,571 1,153 8,318 Revenues Costs and expenses: Cast of sales Selling informational and administrative expenses Research and development expenses Amortization of intangible assets Restructuring charges and certain acquisition-related costs Other deductions-net Income from continuing operations before provision for taxes on income Provision for taxes on income Income from continuing operations Discontinued operations: income/loss) from discontinued operations-net of tax Gain/loss) on sale of discontinued operations-net of tax Discontinued operations-net of tax Net income before allocation to noncontrolling interests Less: Net income attributable to noncontrolling interests Net income attributable to PharmaCorp Inc. Earnings per common share-basic: income from continuing operations attributable to PharmaCorp Inc. common sharehol Discontinued operations-net of tax Net income attributable to PharmaCorp Inc. common shareholders Earnings per common share-diluted: income from continuing operations attributable to PharmaCorp Inc. common sharehold Discontinued operations-net of tax Net income attributable to PharmaCorp Inc.common shareholders Weighted average shares-basic Weighted average shares-diluted Cash dividends paid per common share Page 2 250 1,404 1,654 10,049 -19 -11 -30 8,288 14,598 40 31 28 $14,570 $10,009 $8,257 $127 $0.68 $1.96 $1.07 $0.21 $1.03 $0.00 $1.03 $128 $1.26 $0.68 $1.94 7,442 7,508 $0.88 $1.06 $0 21 $127 7,817 7,870 $0.80 $1.03 $0.00 $1.02 8,036 8,074 $0.72 PharmaCorp Consolidated Statements of Cash Flows (USDS) In Millions, unless otherwise specified Dec. 31, 2015 Dec 31, 2014 Dec 31, 2013 (audited) (audited) $10,049 $8.288 8,809 519 1,298 -1,688 207 147 -1,769 -172 8,299 505 3,386 111 2,109 -156 -677 49 -66 1,184 801 -367 1,498 -708 2,917 -718 401 1.214 -12.666 11,454 20,240 (unaudited) Operating Activities Net income before allocation to noncontrolling interests $14,598 Adjustments to reconcile net income before allocation to noncontrolling interests to net cash provided by operating activities: Depreciation and amortization 7,711 Share-based compensation expense 381 Asset write-offs and impairment charges 1,199 Gain/loss on sale of discontinued operations -7,123 Deferred taxes from continuing operations 839 Deferred taxes from discontinued operations 1,459 Benefit plan contributions (in excess of)/less than expense 135 Other non-cash adjustments, net -203 Other changes in assets and liabilities, net of acquisitions and divestitures: Accounts receivable 375 Inventories -731 Other assets 93 Accounts payable 569 Other liabilities -3.438 Other tax accounts, net 1,190 Net cash provided by operating activities 17,054 Investing Activities Purchases of property, plant and equipment -1.327 Purchases of short-term investments -24,018 Proceeds from redemptions and sales of short-term investments 25,302 Net proceeds from redemptions and sales of short-term investments with original matu 1.459 Purchases of long-term investments -11,145 Proceeds from redemptions and sales of long-term investments 4,990 Acquisitions, net of cash acquired -1,050 Proceeds from sale of businesses 11,850 Other investing activities 93 Net cash provided by/(used in) investing activities 6,154 Financing Activities Proceeds from short-term borrowings 7,985 Principal payments on short-term borrowings 7 Net payments on short-term borrowings with original maturities of 90 days or less -8,304 Principal payments on long-term debt -1,413 Purchases of common stock -8,228 Cash dividends paid -6,534 Other financing activities Net cash used in financing activities -15,999 Effect of exchange rate changes on cash and cash equivalents Net increase/decrease) in cash and cash equivalents 7,207 Cash and cash equivalents, beginning 3,182 Cash and cash equivalents, ending 10,389 Cash paid during the period for: Income taxes 2,430 Interest $1,873 -1.660 -18.447 14,176 10,874 -4,620 2,147 -3,282 2,376 279 1,843 -1.513 -11,082 5,699 5,950 -4,128 4,737 -273 0 118 492 6,500 -1,197 -106 -1,000 -6,088 12,910 -3,926 -7,540 -6,896 -9,100 -6,134 169 20,607 -29 1,447 1,735 3,182 66 -11,174 -31 -243 1,978 1,735 2,938 $2.085 11,775 $2.155 PharmaCorp Operating Segments We manage our operations through five operating segments - Primary Care; Specialty Care and Oncology; Established Products and Emerging Markets; Animal Health; and Consumer Healthcare. As of the third quarter of 2012, the Animal Health and Consumer Healthcare business units are no longer managed as a single operating segment. Each operating segment has responsibility for its commercial activities and for certain research and development activities related to in-line products and in-process research and development (IPR&D) projects that generally have achieved proof-of-concept. On November 30, 2015, we completed the sale of our Nutrition business to Choco and recognized a gain on the sale of this business in Gain/Closs) on sale of discontinued operations-net of tax in the consolidated statement of income for the year ended December 31, 2015. The operating results of this business are reported as Income/(loss) from discontinued operations net of tax in the consolidated statements of income for all periods presented. We regularly review our segments and the approach used by management to evaluate performance and allocate resources. Generally, products are transferred to the Established Products business unit in the beginning of the fiscal year following loss of patent protection or marketing exclusivity A description of each of our five operating segments follows: Primary Care operating segment -includes revenues from human prescription pharmaceutical products primarily prescribed by primary care physicians, and may include products in the following therapeutic and disease areas: Alzheimer's disease, cardiovascular (excluding pulmonary arterial hypertension), erectile dysfunction, genitourinary, major depressive disorder, pain, respiratory and smoking cessation. All revenues for these products are allocated to the Primary Care business unit, except those generated in emerging markets and those that are managed by the Established Products business unit. Specialty Care and Oncology operating segment -comprises the Specialty Care business unit and the Oncology business unit. Specialty Care-includes revenues from human prescription pharmaceutical products primarily prescribed by physicians who are specialists, and may include products in the following therapeutic and disease areas: anti-infectives, endocrine disorders, hemophilia, inflammation, ophthalmology, pulmonary arterial hypertension, specialty neuroscience and vaccines. All revenues for these products are allocated to the Specialty Care business unit, except those generated in emerging markets and those that are managed by the Established Products business unit. Oncologyincludes revenues from human prescription pharmaceutical products addressing oncology and oncology-related illnesses. All revenues for these products are allocated to the Oncology business unit, except those generated in emerging markets and those that are managed by the Established Products business unit. Established Products and Emerging Markets operating segment comprises the Established Products business unit and the Emerging Markets business unit. Established Products includes revenues from human prescription pharmaceutical products that have lost patent protection or marketing exclusivity in certain countries and/or regions. Typically, products are transferred to this business unit in the beginning of the fiscal year following loss of patent protection or marketing exclusivity. However, in certain situations, products may be transferred to this business unit at a different point than the beginning of the fiscal year following loss of patent protection or marketing exclusivity in order to maximize their value. This business unit also excludes revenues generated in emerging markets. Emerging Markets-includes revenues from all human prescription pharmaceutical products sold in emerging markets, including Asia (excluding Japan and South Korea), Latin America, the Middle East, Eastern Europe, Africa, Turkey and Central Europe. PharmaCorp Geographic Information Revenues exceeded $500 million in each of 16 countries outside the U.S. in 2015 and 2014, and in each of 17 countries outside the U.S. in 2013. The U.S. and Japan were the only countries to contribute more than 10% of total revenue in 2015. The U.S. was the only country to contribute more than 10% of total revenue in 2014 and 2013. The following table provides revenues by geographic area: Year Ended December 31, 2015 2014 2013 (MILLIONS OF DOLLARS) Revenues United States $ 23,086 $ 26,933 $ 28,855 Developed Europe 13,375 16,099 16,156 Developed Rest of World 10,554 10,975 9,891 Emerging Markets 11,971 11,252 10,263 Revenues $ 58,986 $ 65,259 $ 65,165 PharmaCorp Other Revenue Information Significant Customers: We sell our products primarily to customers in the wholesale sector. In 2015, sales to our three largest U.S. wholesaler customers represented approximately 12%, 9% and 7% of total revenues and, collectively, represented approximately 16% of total accounts receivable as of December 31, 2015. In 2014, sales to our three largest U.S. wholesaler customers represented approximately 13%, 11% and 9% of total revenues and collectively, represented approximately 14% of total accounts receivable as of December 31, 2014. For both years, these sales and related accounts receivable were concentrated in our three biopharmaceutical operating segments. Significant Product Revenues The following table provides revenues by product: Year Ended December 31, (MILLIONS OF DOLLARS) 2015 2014 2013 Total revenues from biopharmaceutical products 51,214 57,747 58,523 Revenues from other products: Animal Health 4,299 4,184 3,575 Consumer Healthcare 3,212 3,028 2,748 Other 261 300 319 Revenues $ 58,986 $ 65,259 $ 65,165 Top Five Products in Terms of Revenue (all biopharmaceutical products) Year Ended December 31, (MILLIONS OF DOLLARS) 2015 2014 2013 Lyran $ 4,158 $ 3,693 $ 3,063 Lipco 3,948 9,577 10,733 Enbing (Outside the U.S. and Canada) 3,737 3,666 3,274 Prevnent 3,718 3,657 2,416 Selebrax 2,719 2,523 2,374 PharmaCorp Long-Term Debt The following table povides the company of mir unsecured long-term ddat As of December 31. Munty Dute 2015 2014 6.20% Mach 2022 3.348 5.35% M-18 2.965 2.969 7.20% M42 2.803 2.848 4.75% 2,738 2.683 5.7556 Jun-24 2.734 2.581 3.625% December 31 2015, the note haben reclado www Jun-16 - 2.392 6.50% Jun-41 2.206 Page:1 5.95% Ap 40 2.188 5.50% Fdy 2017 1.832 5.50% December 2015, the mots had been called and in utstanding) M-16 - 1,564 4.55% May-20 1.425 4.75% Thaaha 2017 1.294 1.166 5.50% Fo-19 1,048 1,061 6.51% 2028 3,403 3.345 5.28% 2019 2.254 2.402 2018 771 865 Longer debe 31.036 34.926 20-F FILINGS NOVARTELL GROUP CONSOLIDATED FINANCIAL STATEMENTS CONSOLIDATED BALANCE STIFTS At Der 31, 2015 2014) 2013 2011 Nascara Propeaty plant & equipment Goodwill Intangible and other than goodwill HvairamE in metal Dead 16,839 31.190 30,431 8,940 7.190 1.117 505 15.727 29.843 31,869 8,722 5,957 938 Other non- 456 Tatal -cara 96.212 93,412 Carrata Trademables Marketable securities and derivative financial instrummix Cash and chequivals Other 6.844 10.051 2.667 5,652 2.990 5.830 10.432 1.466 3,609 2.756 Tatal current 28,004 24.084 Tatalte 124,216 117.496 Equity and liabilid Equity Sha capital Truy khai Page 1 1.016 0.001 092) (121) 64,949 69.093 65.844 Iwwe share capital and rasva attributable to sevante AG shareholders Non-controlling interest 126 96 Tatal equity 69,219 65,940 Liabili Nos carril Financial dat Debonds blocs Previsions and other non-cumont while 13.88 7.186 13.055 6,861 7.792 Total arratis billi 30,946 28.408 4.898 Curral liabilities Trade payable Financial ddat and darivative financial instruments Cummin.com www Provisions and other cummt abilities 5,693 5.845 2.070 10.443 1,706 10.079 Total current bili 24.051 Total liabili 54,999 51.356 Total equity and liabil 124.216 117.496 NOVARTELL GROUP CONSOLIDATED FINANCIAL STATEMENTS CONSOLIDATED INCOME STATEMENTS (For the years ended December 31, 2015, 2014 and 2013) 2014 (audited) 2015 (unaudited) Sm 56,773 2013 (audited) Sm Net sales Other revenues Cost of goods sold 50,724 $37 909 (18,756) 40,392 (15,179) Grex profit Marketing & Sales Research & Development General & Administration Other income Other expertise 38.805 (14,353) (9,432) (2.837) Page 2 37,073 (13,416) (8,970) (2.481) 1.214 (1,914) (2.870) 1.254 (3,116) 11.11 652 Operating income Income from associated companies Interest expense Other financial income and expense 11.526 904 -792 -851 (2 (96) Income before taxes Taxes 11,243 (1,625 ) 10.773 (1,528 11,702 (1,733 Net income 9,618 9,999 Adriables Shareholders of vartell AG Nor-controlling interests Bask earnings per share (5) Diluted earnings per share (5) 9,505 113 3.93 3.89 9,113 132 3.83 3.78 9,794 175 4.28 4.26 NOVARTELL GROUP CONSOLIDATED FINANCIAL STATEMENTS CONSOLIDATED CASH FLOW STATEMENTS (For the year ended December 31, 2015, 2014 and 2013) 20141 S. S. S. Nutcome Reval of non-white Dividends received from cisted companies and other Intel Intl Other financial reaccipbx Other inci wym Taxes 9.6IN 7.938 326 149 (594) 9.243 9.200 304 66 9.969 6.262 471 180 -535) (6:40) (22) 2.022) (145) 2.616) (2.435) 15.909 15,893 13,586 Gash saw bere werking capital and provixisa changa Restructuring paymmix and other cah payments from pavi Change in net cum and other opening wh flow ta (1.173 ) (1.471) (1.281) (140) (113) 1,762 Cash daw from operating this 14,194 14,309 14.069 2.698) 92 3701 263 (2.167) 161 (20) 543 (139) 49 Page 3 Purchase of pepty.pl Procoads from alex of pepty plus & quit Purchase of the Proceeds from all of inblad Purchase official was Proceeds from alle officia Purchase of the non-cumont Proceeds from alex of other non-contacts Acquisitions of interats in excited compania Acquisition and divxmix of business Purchase of metable oil Proceeds from all of maktable xocuti (1.678) 46 554) 535 (124) 66 25 13 (1.741) (1.639) 516 15 (12) (569) (1.750 3,345 (26,666 (40,5691 53,200 Cash saw wedisiner 8.675 ) (15.756) (505) 514 1.879 (704) (1.737) 3.628 59 381 811 5.524 Acquisition of our sh Disposal of the Hate in Ha- as Repayment of non-cut debat Change in cum incidat Proceeds from ince of she capital to third parties Acquisition of non-controlling intets Dividendx paid to non-controlling in texts and other financing cah flow Dividend paid to shardholdex of novell AG 2.610 19 16 (3,054 4 (3.187 (203) (86) (64) 16.030) (5,368) (4.486 Cash Bawwe in financing 16.675 ) (15,024) 4.116 Nec efect of cummcy raion on hand cah equivalent (1) (103) Nat change in cash and cash equivalente Cah and ah us| 1.843 3,709 (1.610) 5,319 2.994 Cash and cash equivalente at December 31 5,552 3,709 5,319 20-F FILINGS AstraZoro Consolidated Balance Shoot at 31 December 2015 (unaudited) Sm 2014 (audited) $m 2013 (audited) $m Assets 6,189 9,798 16,348 6,525 9,762 10,880 6,957 9,871 12,158 Non-current assets Property, plant and Cquipment Goodwill Intangible assets Derivative financial instruments Other investments Other roocivables Deferred tax assets 442 201 324 211 489 299 452 1,111 34,486 1,514 29,324 1,475 30,996 2,061 7,629 823 1,852 8,754 4,248 1,682 7,847 1,482 Current assets Inventories Trade and other receivables Other investments Derivative financial instruments Income tax receivable Cash and cash equivalents 31 803 7,701 19,048 53,534 25 1,056 7,571 23,506 52,830 9 3,043 11,068 25,131 56,127 Total assets -801 Liabilities Current liabilities Interest-bearing loans and borrowings Trade and other payables Derivative financial instruments Provisions Income tax payable -1800) 1/75) -9321 ) (125) (8,661) -109 ) -816 (2,862 ) (13,903) (1,388) (3,390) (15,752) (8) (1,095) (6,898) (16,787) -9309) -2676 ) -7438 -2635) (9,097) (3,145 ) Non-current liabilities Interest-bearing loans and borrowings Deferred tax liabilities Retirement benefit obligations Provisions Other payables -2165) -538) (1,001) (15,6 79) (29,582) 23,952 (2,674) (474) (385) (13,6 06) (29,358) 23,472 (2,472 (843) (373) (15,9 30) (32,717) 23,410 Total liabilities Net assets Equity Capital and reserves attributable to equity holders of the Company Share capital Share premium account Capital redemption reserve Merger reserve Other reserves Retained earings 302 3,604 253 533 1,374 17,961 23,737 215 23,952 423 2,978 239 533 1,379 17,894 23,246 226 23,472 352 2,672 107 433 1,377 18,272 23,213 197 23,410 Non-controlling interests Total equity AstraZoro Consolidated Statements of Income for the year ended 31 December 2015 2014 2013 (unaudited) Sm 27,963 -5383) 22,580 (audited) Sm 33,581 -6016) 27,565 (346) (audited) Sm 33,269 (6,389) 26,880 (335) (320) -5233 ) -5533) (5,318 -9849) -11061) (10,445 Revenue Cost of sales Gross profit Distribution costs Research and development expense Selling, general and administrative costs Profit on disposal of subsidiary Other operating income and expense Operating profit Finance income Finance expense Profit before tax Taxation Profit for the period 1,483 970 8,148 528 (958) 7,718 (1,391) 6,327 777 12,795 552 (980) 12,367 2,351) 10,016 712 11,494 516 (1,033) 10,977 (2,896) 8,081 106 (60) 26 101 Other comprehensive income: Foreign exchange arising on consolidation Foreign exchange differences on borrowings designated in net investment hedges Fair value movement on derivatives designated in met investment hedges Amortisation of loss on cash flow hedge Net available for sale gains taken to equity Actuarial loss for the period Income tax relating to components of other comprehensive income Other comprehensive income for the period, net of Page 2 72 (85) (741) (46) (46) 198 (61) 78 (546) 25 Total comprehensive income for the period 6,405 9,470 8,105 Profit attributable to: Owners of the Parent No controlling interest 9.983 331 8053 28 Total comprehensive income attributable to: Owners of the Parent Non-controlling interests 6,395 10 8058 48 42 $4.99 57.33 55.60 $4.98 S7.30 S5.57 Basic carnings per 50.25 Ordinary Share Diluted earnings per 50.25 Ordinary Share Weighted average number of Ordinary Shares in issue millions) Diluted weighted average number of Ordinary Shares in issue (millions 1,261 1,361 1,438 1,264 1,367 1.446 3,752 3.494 Dividendis declared and paid in the period 3.619 All activities were in respect of continuing operations mmeans millions of US dollars. straZoro Consolidated Statements of Cash Flows For the year ended 31 December 2015 (umandited) Sm 2014 (audited Sm 2013 (audited) Sm 7,718 530 12 367 58 10.977 517 2,528 2.650 2,741 Cash flows from operating activities Profit before tax Finance income and expense Depreciation, amortisation and impairment Decrease increase in trade and other receivables (Increase) decrease inventories (Decrease increase in trade and other payables and provisions 765 (1,108) 10 -160 (256) (1,311) 467 (16) Profit on disposal of subsidiary (1.483) (424) (597) (463) 9,536 13.854 Non-cash and other movements Cash generated from operations Interest paid Tax paid Net cash inflow from operating activities (545) 12.168 (548) (3,999) (2.533) 6,945 7.821 10,680 Cash flows from investing activities Acquisitions of business operations (1,187) (348 3,719 -2843) (125) (672) -739) (791 (3,947 Movement in short-term investments and fixed deposits Purchase of property, plant and equipment Disposal of property, plant and equipment Purchase of intangible assets Disposal of intangible assets Purchase of non current asset investments Disposal of non-current investments Net cash received on disposal of subsidiary Dividends received Interest received 83 (1.390) 210 Page 3 (46) (11) (34) 1,772 145 171 174 (20) (16) (10) Payments made by subsidiaries to non controlling interest Net cash outflow from investing activities Net cash inflow before financing activities (1.859) (2002) (2.226 5,089 5,799 8.454 429 (2.635) 409 (6.015 494 (2.604) 1,980 (1.750) (3.7641 (114 Cash flows from financing activities Proceeds from issue of share capital Repurchase of shares Repayment of obligations under finance leases Issue of loan Repayment of loans Dividendspaid Hedge contracts relating to dividend payments Movement in short-term borrowings Net cash outflow from financing activities Net increase decrease) in cash and cash equivalents in the period Cash and cash equivalents at the beginning of the period Exchange rate effects Cash and cash equivalents at the end of the period (8) (4,923) (9,121) (7114) 166 (3.5221 1,120 7,434 (4) 10,981 (25) 9.828 33 7,596 7,434 10,981 Pharma Corp Consolidated Balance Sheets (USDS) In Millions, except Share data, unless otherwise specified Dec 31, 2015 Dec 31, 2014 (unaudited) (audited) ASSETS Cash and cash equivalents Short-term investments Accounts receivable, less allowance for doubtful accounts, 2015-$374;2014-$226 Inventories Taxes and other current assets Assets of discontinued operations and other assets held for sale Total current assets Long-term investments Property, plant and equipment, less accumulated depreciation Goodwill Identifiable intangible assets, less accumulated amortization Taxes and other noncurrent assets Total assets $10,489 22,219 12,478 6,963 9,196 70 61,415 14.249 14,361 44,572 46,113 5.088 185,798 $3,282 23,170 13,158 6,510 9,480 5.217 60,917 9,914 15,921 44,669 51,284 5,697 188 6,524 4,116 4,164 LIABILITIES AND EQUITIES Short-term borrowings, including current portion of long-term debt: 2015-$2,449; 2014-$6 Accounts payable Dividends payable Income taxes payable Accrued compensation and related items Other current liabilities Liabilities of discontinued operations Total current liabilities Long-term debt Pension benefit obligations Postretirement benefit obligations Noncurrent deferred tax liabilities Other taxes payable Other noncurrent liabilities Total liabilities 910 2.146 13.041 0 28,719 30,936 7,930 3,393 21,593 6,610 4,939 104,120 3,578 1,896 909 2,220 15,066 1,124 28,909 34,826 6,455 3,244 18,861 6,886 6,100 105,381 39 45 445 Preferred stack, without par value, at stated value: 27 shares authorized; issued: 2015967,2014-1,112 Common stock, $0.05 par value: 12,000 shares authorized; issued: 2015-8,956; 2014-8,902 Additional paid-in capital Employee benefit trusts Treasury stack, shares at cost: 2015-1,680; 2014-1,327 Retained earnings Accumulated other comprehensive loss Total shareholders' equity Equity attributable to noncontrolling interests Total equity 72,608 -1 -40,121 54,240 -5,953 81,260 418 81,678 71,423 -3 -31,801 46,210 -4,129 82,190 431 82.621 Total liabilities and equity 185,798 188,002 PharmaCorp Consolidated Statements of Income (USDS) In Millions, except Per Share data, unless otherwise specified Dec 31, 2015 Dec 31, 2014 Dec. 31, 2013 $58,886 $65,159 $65,065 11,234 16,516 7,770 5,275 1,880 4,031 12,080 2.462 9,618 14,176 18,632 9,074 5,644 2,830 2,499 12,304 3,809 8,495 14,888 18,873 9,583 5,264 3,245 3,841 9,571 1,153 8,318 Revenues Costs and expenses: Cast of sales Selling informational and administrative expenses Research and development expenses Amortization of intangible assets Restructuring charges and certain acquisition-related costs Other deductions-net Income from continuing operations before provision for taxes on income Provision for taxes on income Income from continuing operations Discontinued operations: income/loss) from discontinued operations-net of tax Gain/loss) on sale of discontinued operations-net of tax Discontinued operations-net of tax Net income before allocation to noncontrolling interests Less: Net income attributable to noncontrolling interests Net income attributable to PharmaCorp Inc. Earnings per common share-basic: income from continuing operations attributable to PharmaCorp Inc. common sharehol Discontinued operations-net of tax Net income attributable to PharmaCorp Inc. common shareholders Earnings per common share-diluted: income from continuing operations attributable to PharmaCorp Inc. common sharehold Discontinued operations-net of tax Net income attributable to PharmaCorp Inc.common shareholders Weighted average shares-basic Weighted average shares-diluted Cash dividends paid per common share Page 2 250 1,404 1,654 10,049 -19 -11 -30 8,288 14,598 40 31 28 $14,570 $10,009 $8,257 $127 $0.68 $1.96 $1.07 $0.21 $1.03 $0.00 $1.03 $128 $1.26 $0.68 $1.94 7,442 7,508 $0.88 $1.06 $0 21 $127 7,817 7,870 $0.80 $1.03 $0.00 $1.02 8,036 8,074 $0.72 PharmaCorp Consolidated Statements of Cash Flows (USDS) In Millions, unless otherwise specified Dec. 31, 2015 Dec 31, 2014 Dec 31, 2013 (audited) (audited) $10,049 $8.288 8,809 519 1,298 -1,688 207 147 -1,769 -172 8,299 505 3,386 111 2,109 -156 -677 49 -66 1,184 801 -367 1,498 -708 2,917 -718 401 1.214 -12.666 11,454 20,240 (unaudited) Operating Activities Net income before allocation to noncontrolling interests $14,598 Adjustments to reconcile net income before allocation to noncontrolling interests to net cash provided by operating activities: Depreciation and amortization 7,711 Share-based compensation expense 381 Asset write-offs and impairment charges 1,199 Gain/loss on sale of discontinued operations -7,123 Deferred taxes from continuing operations 839 Deferred taxes from discontinued operations 1,459 Benefit plan contributions (in excess of)/less than expense 135 Other non-cash adjustments, net -203 Other changes in assets and liabilities, net of acquisitions and divestitures: Accounts receivable 375 Inventories -731 Other assets 93 Accounts payable 569 Other liabilities -3.438 Other tax accounts, net 1,190 Net cash provided by operating activities 17,054 Investing Activities Purchases of property, plant and equipment -1.327 Purchases of short-term investments -24,018 Proceeds from redemptions and sales of short-term investments 25,302 Net proceeds from redemptions and sales of short-term investments with original matu 1.459 Purchases of long-term investments -11,145 Proceeds from redemptions and sales of long-term investments 4,990 Acquisitions, net of cash acquired -1,050 Proceeds from sale of businesses 11,850 Other investing activities 93 Net cash provided by/(used in) investing activities 6,154 Financing Activities Proceeds from short-term borrowings 7,985 Principal payments on short-term borrowings 7 Net payments on short-term borrowings with original maturities of 90 days or less -8,304 Principal payments on long-term debt -1,413 Purchases of common stock -8,228 Cash dividends paid -6,534 Other financing activities Net cash used in financing activities -15,999 Effect of exchange rate changes on cash and cash equivalents Net increase/decrease) in cash and cash equivalents 7,207 Cash and cash equivalents, beginning 3,182 Cash and cash equivalents, ending 10,389 Cash paid during the period for: Income taxes 2,430 Interest $1,873 -1.660 -18.447 14,176 10,874 -4,620 2,147 -3,282 2,376 279 1,843 -1.513 -11,082 5,699 5,950 -4,128 4,737 -273 0 118 492 6,500 -1,197 -106 -1,000 -6,088 12,910 -3,926 -7,540 -6,896 -9,100 -6,134 169 20,607 -29 1,447 1,735 3,182 66 -11,174 -31 -243 1,978 1,735 2,938 $2.085 11,775 $2.155 PharmaCorp Operating Segments We manage our operations through five operating segments - Primary Care; Specialty Care and Oncology; Established Products and Emerging Markets; Animal Health; and Consumer Healthcare. As of the third quarter of 2012, the Animal Health and Consumer Healthcare business units are no longer managed as a single operating segment. Each operating segment has responsibility for its commercial activities and for certain research and development activities related to in-line products and in-process research and development (IPR&D) projects that generally have achieved proof-of-concept. On November 30, 2015, we completed the sale of our Nutrition business to Choco and recognized a gain on the sale of this business in Gain/Closs) on sale of discontinued operations-net of tax in the consolidated statement of income for the year ended December 31, 2015. The operating results of this business are reported as Income/(loss) from discontinued operations net of tax in the consolidated statements of income for all periods presented. We regularly review our segments and the approach used by management to evaluate performance and allocate resources. Generally, products are transferred to the Established Products business unit in the beginning of the fiscal year following loss of patent protection or marketing exclusivity A description of each of our five operating segments follows: Primary Care operating segment -includes revenues from human prescription pharmaceutical products primarily prescribed by primary care physicians, and may include products in the following therapeutic and disease areas: Alzheimer's disease, cardiovascular (excluding pulmonary arterial hypertension), erectile dysfunction, genitourinary, major depressive disorder, pain, respiratory and smoking cessation. All revenues for these products are allocated to the Primary Care business unit, except those generated in emerging markets and those that are managed by the Established Products business unit. Specialty Care and Oncology operating segment -comprises the Specialty Care business unit and the Oncology business unit. Specialty Care-includes revenues from human prescription pharmaceutical products primarily prescribed by physicians who are specialists, and may include products in the following therapeutic and disease areas: anti-infectives, endocrine disorders, hemophilia, inflammation, ophthalmology, pulmonary arterial hypertension, specialty neuroscience and vaccines. All revenues for these products are allocated to the Specialty Care business unit, except those generated in emerging markets and those that are managed by the Established Products business unit. Oncologyincludes revenues from human prescription pharmaceutical products addressing oncology and oncology-related illnesses. All revenues for these products are allocated to the Oncology business unit, except those generated in emerging markets and those that are managed by the Established Products business unit. Established Products and Emerging Markets operating segment comprises the Established Products business unit and the Emerging Markets business unit. Established Products includes revenues from human prescription pharmaceutical products that have lost patent protection or marketing exclusivity in certain countries and/or regions. Typically, products are transferred to this business unit in the beginning of the fiscal year following loss of patent protection or marketing exclusivity. However, in certain situations, products may be transferred to this business unit at a different point than the beginning of the fiscal year following loss of patent protection or marketing exclusivity in order to maximize their value. This business unit also excludes revenues generated in emerging markets. Emerging Markets-includes revenues from all human prescription pharmaceutical products sold in emerging markets, including Asia (excluding Japan and South Korea), Latin America, the Middle East, Eastern Europe, Africa, Turkey and Central Europe. PharmaCorp Geographic Information Revenues exceeded $500 million in each of 16 countries outside the U.S. in 2015 and 2014, and in each of 17 countries outside the U.S. in 2013. The U.S. and Japan were the only countries to contribute more than 10% of total revenue in 2015. The U.S. was the only country to contribute more than 10% of total revenue in 2014 and 2013. The following table provides revenues by geographic area: Year Ended December 31, 2015 2014 2013 (MILLIONS OF DOLLARS) Revenues United States $ 23,086 $ 26,933 $ 28,855 Developed Europe 13,375 16,099 16,156 Developed Rest of World 10,554 10,975 9,891 Emerging Markets 11,971 11,252 10,263 Revenues $ 58,986 $ 65,259 $ 65,165 PharmaCorp Other Revenue Information Significant Customers: We sell our products primarily to customers in the wholesale sector. In 2015, sales to our three largest U.S. wholesaler customers represented approximately 12%, 9% and 7% of total revenues and, collectively, represented approximately 16% of total accounts receivable as of December 31, 2015. In 2014, sales to our three largest U.S. wholesaler customers represented approximately 13%, 11% and 9% of total revenues and collectively, represented approximately 14% of total accounts receivable as of December 31, 2014. For both years, these sales and related accounts receivable were concentrated in our three biopharmaceutical operating segments. Significant Product Revenues The following table provides revenues by product: Year Ended December 31, (MILLIONS OF DOLLARS) 2015 2014 2013 Total revenues from biopharmaceutical products 51,214 57,747 58,523 Revenues from other products: Animal Health 4,299 4,184 3,575 Consumer Healthcare 3,212 3,028 2,748 Other 261 300 319 Revenues $ 58,986 $ 65,259 $ 65,165 Top Five Products in Terms of Revenue (all biopharmaceutical products) Year Ended December 31, (MILLIONS OF DOLLARS) 2015 2014 2013 Lyran $ 4,158 $ 3,693 $ 3,063 Lipco 3,948 9,577 10,733 Enbing (Outside the U.S. and Canada) 3,737 3,666 3,274 Prevnent 3,718 3,657 2,416 Selebrax 2,719 2,523 2,374 PharmaCorp Long-Term Debt The following table povides the company of mir unsecured long-term ddat As of December 31. Munty Dute 2015 2014 6.20% Mach 2022 3.348 5.35% M-18 2.965 2.969 7.20% M42 2.803 2.848 4.75% 2,738 2.683 5.7556 Jun-24 2.734 2.581 3.625% December 31 2015, the note haben reclado www Jun-16 - 2.392 6.50% Jun-41 2.206 Page:1 5.95% Ap 40 2.188 5.50% Fdy 2017 1.832 5.50% December 2015, the mots had been called and in utstanding) M-16 - 1,564 4.55% May-20 1.425 4.75% Thaaha 2017 1.294 1.166 5.50% Fo-19 1,048 1,061 6.51% 2028 3,403 3.345 5.28% 2019 2.254 2.402 2018 771 865 Longer debe 31.036 34.926 20-F FILINGS NOVARTELL GROUP CONSOLIDATED FINANCIAL STATEMENTS CONSOLIDATED BALANCE STIFTS At Der 31, 2015 2014) 2013 2011 Nascara Propeaty plant & equipment Goodwill Intangible and other than goodwill HvairamE in metal Dead 16,839 31.190 30,431 8,940 7.190 1.117 505 15.727 29.843 31,869 8,722 5,957 938 Other non- 456 Tatal -cara 96.212 93,412 Carrata Trademables Marketable securities and derivative financial instrummix Cash and chequivals Other 6.844 10.051 2.667 5,652 2.990 5.830 10.432 1.466 3,609 2.756 Tatal current 28,004 24.084 Tatalte 124,216 117.496 Equity and liabilid Equity Sha capital Truy khai Page 1 1.016 0.001 092) (121) 64,949 69.093 65.844 Iwwe share capital and rasva attributable to sevante AG shareholders Non-controlling interest 126 96 Tatal equity 69,219 65,940 Liabili Nos carril Financial dat Debonds blocs Previsions and other non-cumont while 13.88 7.186 13.055 6,861 7.792 Total arratis billi 30,946 28.408 4.898 Curral liabilities Trade payable Financial ddat and darivative financial instruments Cummin.com www Provisions and other cummt abilities 5,693 5.845 2.070 10.443 1,706 10.079 Total current bili 24.051 Total liabili 54,999 51.356 Total equity and liabil 124.216 117.496 NOVARTELL GROUP CONSOLIDATED FINANCIAL STATEMENTS CONSOLIDATED INCOME STATEMENTS (For the years ended December 31, 2015, 2014 and 2013) 2014 (audited) 2015 (unaudited) Sm 56,773 2013 (audited) Sm Net sales Other revenues Cost of goods sold 50,724 $37 909 (18,756) 40,392 (15,179) Grex profit Marketing & Sales Research & Development General & Administration Other income Other expertise 38.805 (14,353) (9,432) (2.837) Page 2 37,073 (13,416) (8,970) (2.481) 1.214 (1,914) (2.870) 1.254 (3,116) 11.11 652 Operating income Income from associated companies Interest expense Other financial income and expense 11.526 904 -792 -851 (2 (96) Income before taxes Taxes 11,243 (1,625 ) 10.773 (1,528 11,702 (1,733 Net income 9,618 9,999 Adriables Shareholders of vartell AG Nor-controlling interests Bask earnings per share (5) Diluted earnings per share (5) 9,505 113 3.93 3.89 9,113 132 3.83 3.78 9,794 175 4.28 4.26 NOVARTELL GROUP CONSOLIDATED FINANCIAL STATEMENTS CONSOLIDATED CASH FLOW STATEMENTS (For the year ended December 31, 2015, 2014 and 2013) 20141 S. S. S. Nutcome Reval of non-white Dividends received from cisted companies and other Intel Intl Other financial reaccipbx Other inci wym Taxes 9.6IN 7.938 326 149 (594) 9.243 9.200 304 66 9.969 6.262 471 180 -535) (6:40) (22) 2.022) (145) 2.616) (2.435) 15.909 15,893 13,586 Gash saw bere werking capital and provixisa changa Restructuring paymmix and other cah payments from pavi Change in net cum and other opening wh flow ta (1.173 ) (1.471) (1.281) (140) (113) 1,762 Cash daw from operating this 14,194 14,309 14.069 2.698) 92 3701 263 (2.167) 161 (20) 543 (139) 49 Page 3 Purchase of pepty.pl Procoads from alex of pepty plus & quit Purchase of the Proceeds from all of inblad Purchase official was Proceeds from alle officia Purchase of the non-cumont Proceeds from alex of other non-contacts Acquisitions of interats in excited compania Acquisition and divxmix of business Purchase of metable oil Proceeds from all of maktable xocuti (1.678) 46 554) 535 (124) 66 25 13 (1.741) (1.639) 516 15 (12) (569) (1.750 3,345 (26,666 (40,5691 53,200 Cash saw wedisiner 8.675 ) (15.756) (505) 514 1.879 (704) (1.737) 3.628 59 381 811 5.524 Acquisition of our sh Disposal of the Hate in Ha- as Repayment of non-cut debat Change in cum incidat Proceeds from ince of she capital to third parties Acquisition of non-controlling intets Dividendx paid to non-controlling in texts and other financing cah flow Dividend paid to shardholdex of novell AG 2.610 19 16 (3,054 4 (3.187 (203) (86) (64) 16.030) (5,368) (4.486 Cash Bawwe in financing 16.675 ) (15,024) 4.116 Nec efect of cummcy raion on hand cah equivalent (1) (103) Nat change in cash and cash equivalente Cah and ah us| 1.843 3,709 (1.610) 5,319 2.994 Cash and cash equivalente at December 31 5,552 3,709 5,319 20-F FILINGS AstraZoro Consolidated Balance Shoot at 31 December 2015 (unaudited) Sm 2014 (audited) $m 2013 (audited) $m Assets 6,189 9,798 16,348 6,525 9,762 10,880 6,957 9,871 12,158 Non-current assets Property, plant and Cquipment Goodwill Intangible assets Derivative financial instruments Other investments Other roocivables Deferred tax assets 442 201 324 211 489 299 452 1,111 34,486 1,514 29,324 1,475 30,996 2,061 7,629 823 1,852 8,754 4,248 1,682 7,847 1,482 Current assets Inventories Trade and other receivables Other investments Derivative financial instruments Income tax receivable Cash and cash equivalents 31 803 7,701 19,048 53,534 25 1,056 7,571 23,506 52,830 9 3,043 11,068 25,131 56,127 Total assets -801 Liabilities Current liabilities Interest-bearing loans and borrowings Trade and other payables Derivative financial instruments Provisions Income tax payable -1800) 1/75) -9321 ) (125) (8,661) -109 ) -816 (2,862 ) (13,903) (1,388) (3,390) (15,752) (8) (1,095) (6,898) (16,787) -9309) -2676 ) -7438 -2635) (9,097) (3,145 ) Non-current liabilities Interest-bearing loans and borrowings Deferred tax liabilities Retirement benefit obligations Provisions Other payables -2165) -538) (1,001) (15,6 79) (29,582) 23,952 (2,674) (474) (385) (13,6 06) (29,358) 23,472 (2,472 (843) (373) (15,9 30) (32,717) 23,410 Total liabilities Net assets Equity Capital and reserves attributable to equity holders of the Company Share capital Share premium account Capital redemption reserve Merger reserve Other reserves Retained earings 302 3,604 253 533 1,374 17,961 23,737 215 23,952 423 2,978 239 533 1,379 17,894 23,246 226 23,472 352 2,672 107 433 1,377 18,272 23,213 197 23,410 Non-controlling interests Total equity AstraZoro Consolidated Statements of Income for the year ended 31 December 2015 2014 2013 (unaudited) Sm 27,963 -5383) 22,580 (audited) Sm 33,581 -6016) 27,565 (346) (audited) Sm 33,269 (6,389) 26,880 (335) (320) -5233 ) -5533) (5,318 -9849) -11061) (10,445 Revenue Cost of sales Gross profit Distribution costs Research and development expense Selling, general and administrative costs Profit on disposal of subsidiary Other operating income and expense Operating profit Finance income Finance expense Profit before tax Taxation Profit for the period 1,483 970 8,148 528 (958) 7,718 (1,391) 6,327 777 12,795 552 (980) 12,367 2,351) 10,016 712 11,494 516 (1,033) 10,977 (2,896) 8,081 106 (60) 26 101 Other comprehensive income: Foreign exchange arising on consolidation Foreign exchange differences on borrowings designated in net investment hedges Fair value movement on derivatives designated in met investment hedges Amortisation of loss on cash flow hedge Net available for sale gains taken to equity Actuarial loss for the period Income tax relating to components of other comprehensive income Other comprehensive income for the period, net of Page 2 72 (85) (741) (46) (46) 198 (61) 78 (546) 25 Total comprehensive income for the period 6,405 9,470 8,105 Profit attributable to: Owners of the Parent No controlling interest 9.983 331 8053 28 Total comprehensive income attributable to: Owners of the Parent Non-controlling interests 6,395 10 8058 48 42 $4.99 57.33 55.60 $4.98 S7.30 S5.57 Basic carnings per 50.25 Ordinary Share Diluted earnings per 50.25 Ordinary Share Weighted average number of Ordinary Shares in issue millions) Diluted weighted average number of Ordinary Shares in issue (millions 1,261 1,361 1,438 1,264 1,367 1.446 3,752 3.494 Dividendis declared and paid in the period 3.619 All activities were in respect of continuing operations mmeans millions of US dollars. straZoro Consolidated Statements of Cash Flows For the year ended 31 December 2015 (umandited) Sm 2014 (audited Sm 2013 (audited) Sm 7,718 530 12 367 58 10.977 517 2,528 2.650 2,741 Cash flows from operating activities Profit before tax Finance income and expense Depreciation, amortisation and impairment Decrease increase in trade and other receivables (Increase) decrease inventories (Decrease increase in trade and other payables and provisions 765 (1,108) 10 -160 (256) (1,311) 467 (16) Profit on disposal of subsidiary (1.483) (424) (597) (463) 9,536 13.854 Non-cash and other movements Cash generated from operations Interest paid Tax paid Net cash inflow from operating activities (545) 12.168 (548) (3,999) (2.533) 6,945 7.821 10,680 Cash flows from investing activities Acquisitions of business operations (1,187) (348 3,719 -2843) (125) (672) -739) (791 (3,947 Movement in short-term investments and fixed deposits Purchase of property, plant and equipment Disposal of property, plant and equipment Purchase of intangible assets Disposal of intangible assets Purchase of non current asset investments Disposal of non-current investments Net cash received on disposal of subsidiary Dividends received Interest received 83 (1.390) 210 Page 3 (46) (11) (34) 1,772 145 171 174 (20) (16) (10) Payments made by subsidiaries to non controlling interest Net cash outflow from investing activities Net cash inflow before financing activities (1.859) (2002) (2.226 5,089 5,799 8.454 429 (2.635) 409 (6.015 494 (2.604) 1,980 (1.750) (3.7641 (114 Cash flows from financing activities Proceeds from issue of share capital Repurchase of shares Repayment of obligations under finance leases Issue of loan Repayment of loans Dividendspaid Hedge contracts relating to dividend payments Movement in short-term borrowings Net cash outflow from financing activities Net increase decrease) in cash and cash equivalents in the period Cash and cash equivalents at the beginning of the period Exchange rate effects Cash and cash equivalents at the end of the period (8) (4,923) (9,121) (7114) 166 (3.5221 1,120 7,434 (4) 10,981 (25) 9.828 33 7,596 7,434 10,981






















