Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Referring to below, determine the net rate of radiant exchange, in W, for e=0.8, A = 0.157 m2, Tb = 461 K, Ts =
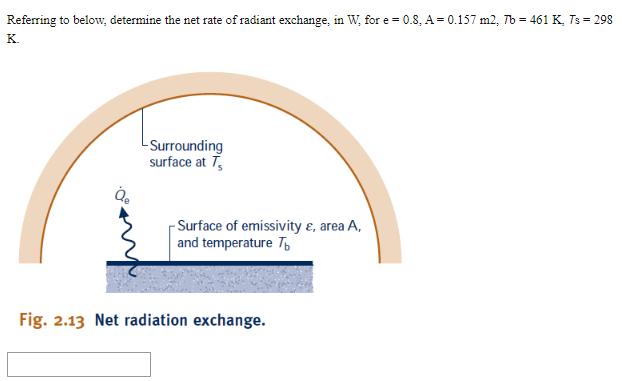
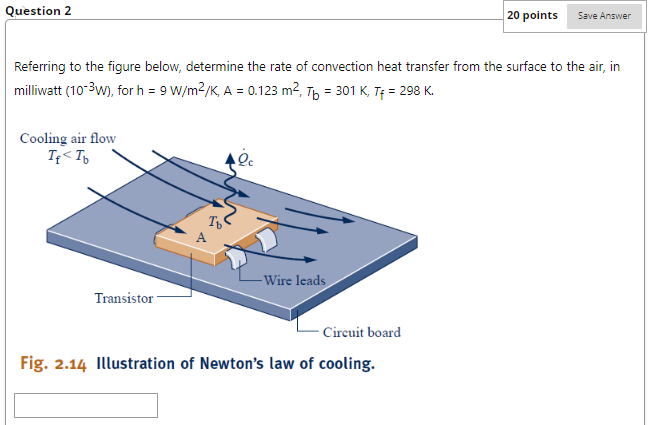


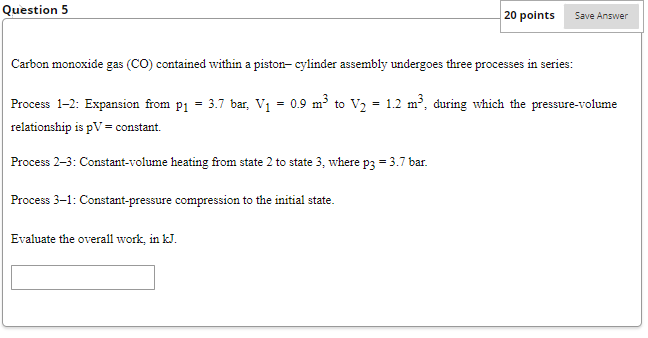
Referring to below, determine the net rate of radiant exchange, in W, for e=0.8, A = 0.157 m2, Tb = 461 K, Ts = 298 K. -Surrounding surface at T -Surface of emissivity &, area A, and temperature T Fig. 2.13 Net radiation exchange. Question 2 Cooling air flow If Question 3 20 points Save Answer A gas is compressed in a piston-cylinder assembly from p = 2.2 bar to p2 = 7.0 bar, V = 0.02 m in a process during which the relation between pressure and volume is pv1.4 = constant. The mass of the gas is 0.4 kg. If the specific internal energy of the gas increases by 40 kJ/kg during the process, determine the heat transfer, in Joules. Kinetic and potential energy changes are negligible. Question 4 20 points Save Answer A mass of 10 kg undergoes a process during which there is heat transfer from the mass at a rate of 5 kJ per kg, an elevation decrease of 47 m, and an increase in velocity from 15 m/s to 27 m/s. The specific internal energy decreases by 5 kJ/kg and the acceleration of gravity is constant at 9.7 m/s2. Determine the work for the process, in Joules. Question 5 20 points Evaluate the overall work, in kJ. Save Answer Carbon monoxide gas (CO) contained within a piston- cylinder assembly undergoes three processes in series: Process 1-2: Expansion from p = 3.7 bar, V = 0.9 m to V = 1.2 m, during which the pressure-volume relationship is pV = constant. Process 2-3: Constant-volume heating from state 2 to state 3, where p3 = 3.7 bar. Process 3-1: Constant-pressure compression to the initial state.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Critically damped system The steadystate error for a critically damped system can be calculated us...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


