Answered step by step
Verified Expert Solution
Question
1 Approved Answer
. Regarding The Difference Between Chemical And Electrochemical Reactions. Which Statement Below Is Not True? (A)During The Electrochemical Reaction, The Electrons Are Released By The
. Regarding The Difference Between Chemical And Electrochemical Reactions. Which Statement Below Is Not True? (A)During The Electrochemical Reaction, The Electrons Are Released By The Oxidation And Transferred Via Electrical Conductor To Places Where Reduction Reactions Occur. (B) Chemical Oxidation And Reduction Occur At The Same Place (C) Electrochemical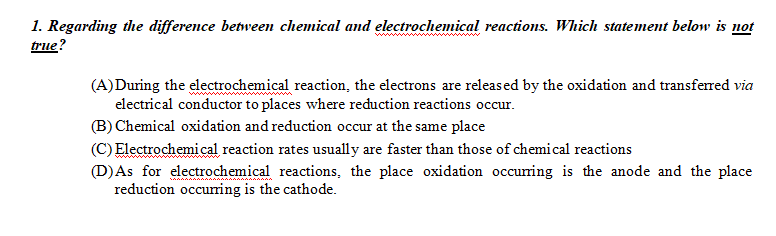
1. Regarding the difference between chemical and electrochemical reactions. Which statement below is not true? (A) During the electrochemical reaction, the electrons are released by the oxidation and transferred via electrical conductor to places where reduction reactions occur. (B) Chemical oxidation and reduction occur at the same place (C) Electrochemical reaction rates usually are faster than those of chemical reactions (D) As for electrochemical reactions, the place oxidation occurring is the anode and the place reduction occurring is the cathode.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


