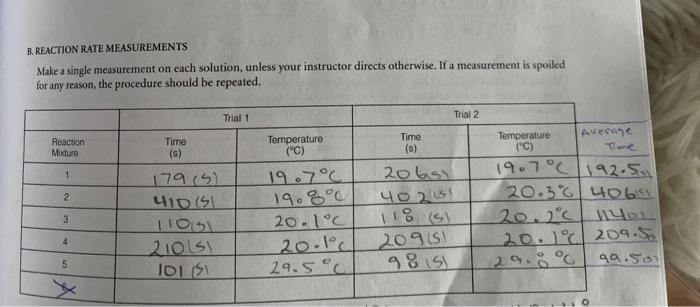
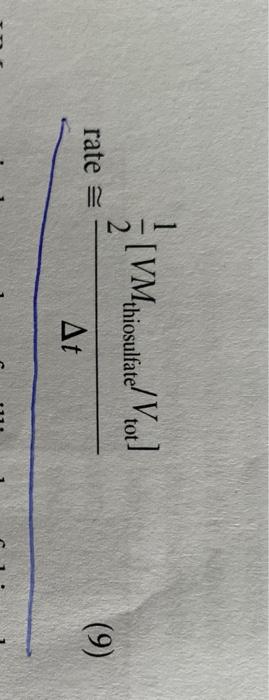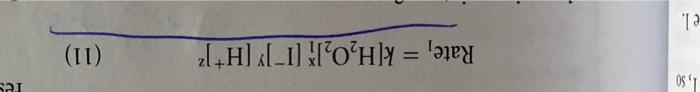REPORT 26 Sheet 3 2. Effect of Temperature po your measurements for Part C.2.confirm the oft-quoted role of thumb that the rate of chemical reaction approximately doubles for every 10 C temperature increase PROBLEMS 1. Considering the volumes and the concentrations used in mixture I. what percentage of the males of H.present have been consumed during the timed reaction? Calculate the number of moles of H., present initially, and determine how many have reacted with iodide when the thiosulfate runs out. How does the concentration change during this same time? Why? 2. Using Equations (9) and (11), calculate the specific rate constant, k, from your data for reaction mixture 1. (Use the integer reaction orders you determined for x, y, and z in the rate expression of Equation [11] Compare your value with the reported value of 0.0115 L.moll.s'at 25 C for the uncatalyzed reaction 3. It has been found that in acid solution (greater than 1 MH') the rate expression for the reaction of H.0, with is of the form: rate = k/H.0,||||[H*] where k, = 0.25 L'. mol. s ' at 25C. Therefore the rate law over the whole range of acidity from very acid solutions to a neutral solution is given by a two-term rate expression: rate=k,(1,0,1") + k, 14.0:||||||*| where k, has the value of 0.0115 L. mol.5 at 25C. Compare the relative magnitudes of these two terms under the conditions employed in your rate measurements for mixture 1. (You should find that the second term makes a negligible contribution to the reaction rate at H* concentrations smaller than 3 x 10 B. REACTION RATE MEASUREMENTS Make a single measurement on each solution, unless your instructor directs otherwise. If a measurement is spoiled for any reason, the procedure should be repeated. Trial 1 Trial 2 Reaction Mixture Time (s) Temperature (C) Time (s) Temperature ("C) 1 19.7C 179(5) 41D (SI 2 Aveca Time . 19.7C/192.50 20.36406 20.2C 140 20.1% 209.50 qa.501 3 20bsi 40208 118 si 20 91 98 is! 20.1C 20.1C 29.5C 210Ls IDIS 5 [VMthiosulfate/Vtot] | Misional rate (9) At mu 1,50 res Rate, = k[H,02H1T][H+)? (11) el. REPORT 26 Sheet 3 2. Effect of Temperature po your measurements for Part C.2.confirm the oft-quoted role of thumb that the rate of chemical reaction approximately doubles for every 10 C temperature increase PROBLEMS 1. Considering the volumes and the concentrations used in mixture I. what percentage of the males of H.present have been consumed during the timed reaction? Calculate the number of moles of H., present initially, and determine how many have reacted with iodide when the thiosulfate runs out. How does the concentration change during this same time? Why? 2. Using Equations (9) and (11), calculate the specific rate constant, k, from your data for reaction mixture 1. (Use the integer reaction orders you determined for x, y, and z in the rate expression of Equation [11] Compare your value with the reported value of 0.0115 L.moll.s'at 25 C for the uncatalyzed reaction 3. It has been found that in acid solution (greater than 1 MH') the rate expression for the reaction of H.0, with is of the form: rate = k/H.0,||||[H*] where k, = 0.25 L'. mol. s ' at 25C. Therefore the rate law over the whole range of acidity from very acid solutions to a neutral solution is given by a two-term rate expression: rate=k,(1,0,1") + k, 14.0:||||||*| where k, has the value of 0.0115 L. mol.5 at 25C. Compare the relative magnitudes of these two terms under the conditions employed in your rate measurements for mixture 1. (You should find that the second term makes a negligible contribution to the reaction rate at H* concentrations smaller than 3 x 10 B. REACTION RATE MEASUREMENTS Make a single measurement on each solution, unless your instructor directs otherwise. If a measurement is spoiled for any reason, the procedure should be repeated. Trial 1 Trial 2 Reaction Mixture Time (s) Temperature (C) Time (s) Temperature ("C) 1 19.7C 179(5) 41D (SI 2 Aveca Time . 19.7C/192.50 20.36406 20.2C 140 20.1% 209.50 qa.501 3 20bsi 40208 118 si 20 91 98 is! 20.1C 20.1C 29.5C 210Ls IDIS 5 [VMthiosulfate/Vtot] | Misional rate (9) At mu 1,50 res Rate, = k[H,02H1T][H+)? (11) el