Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Required intormation The characteristic properties of ethylene are given in the following table: Molar Ve mass IK Po/bar Zo cm3.mol-! IK Ethylene 28.054 0.087 282.3
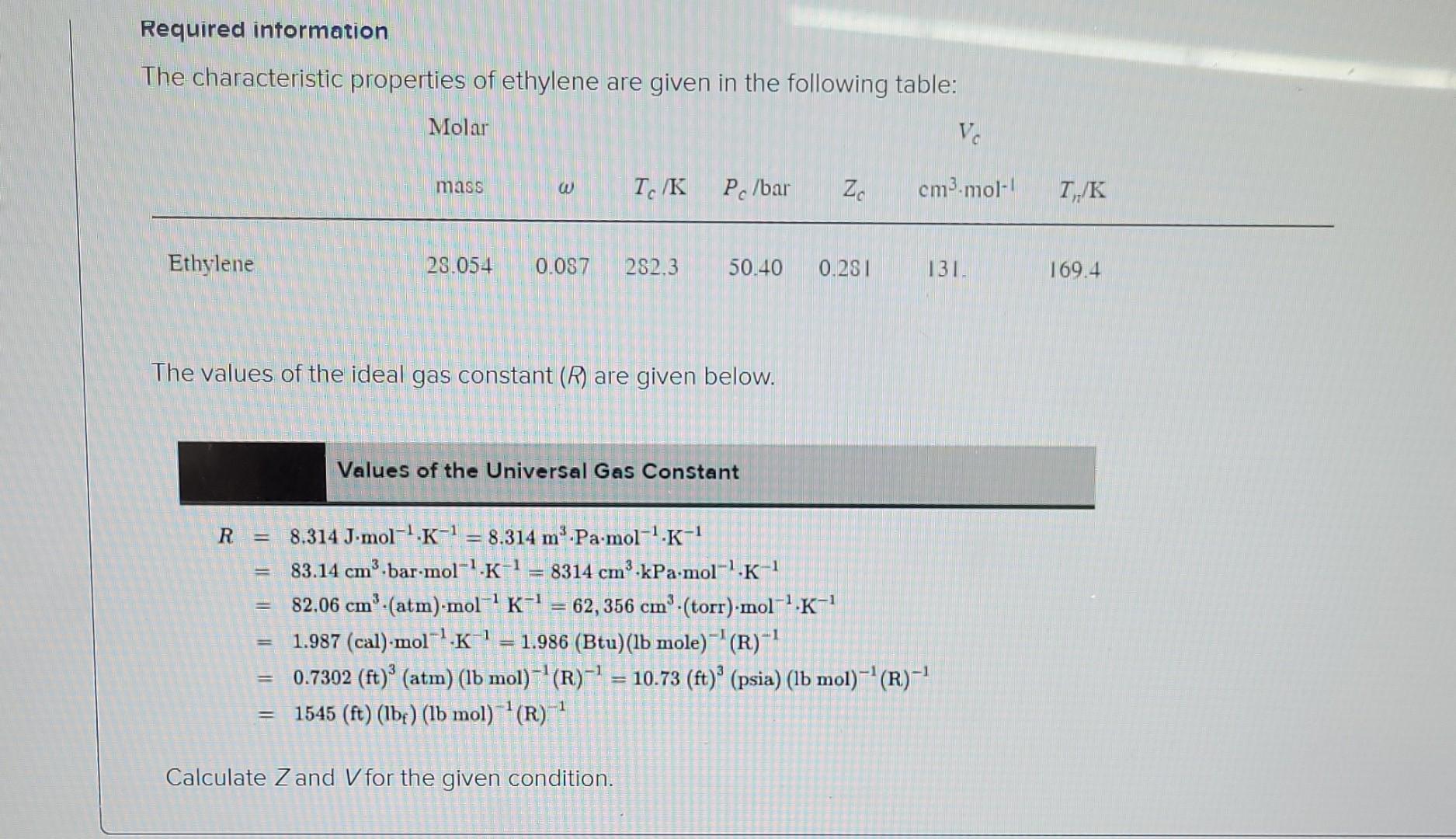
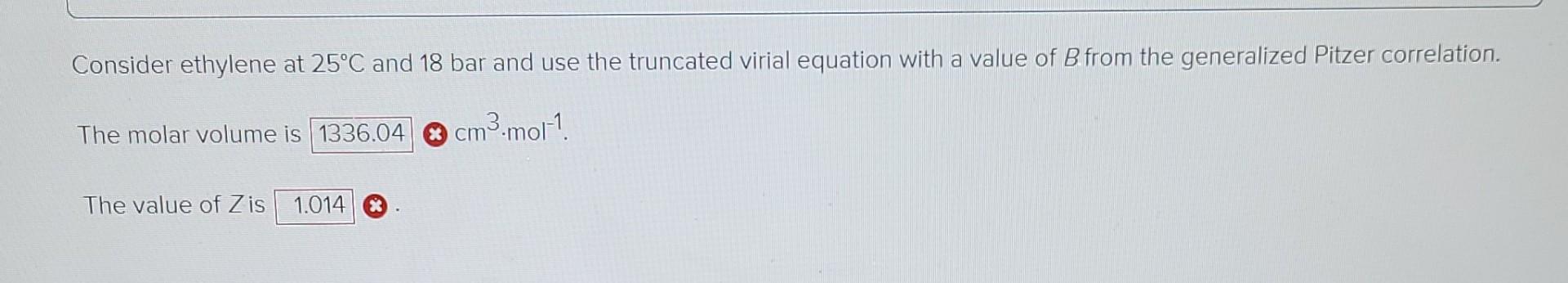
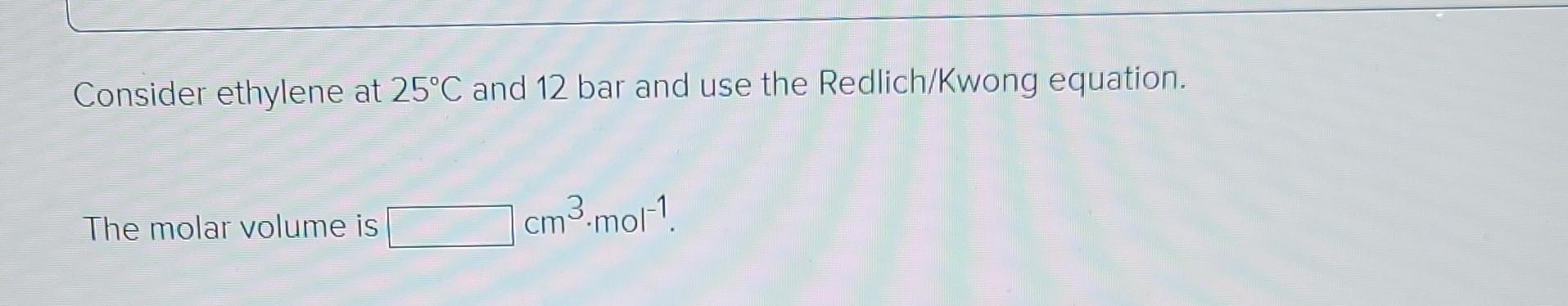
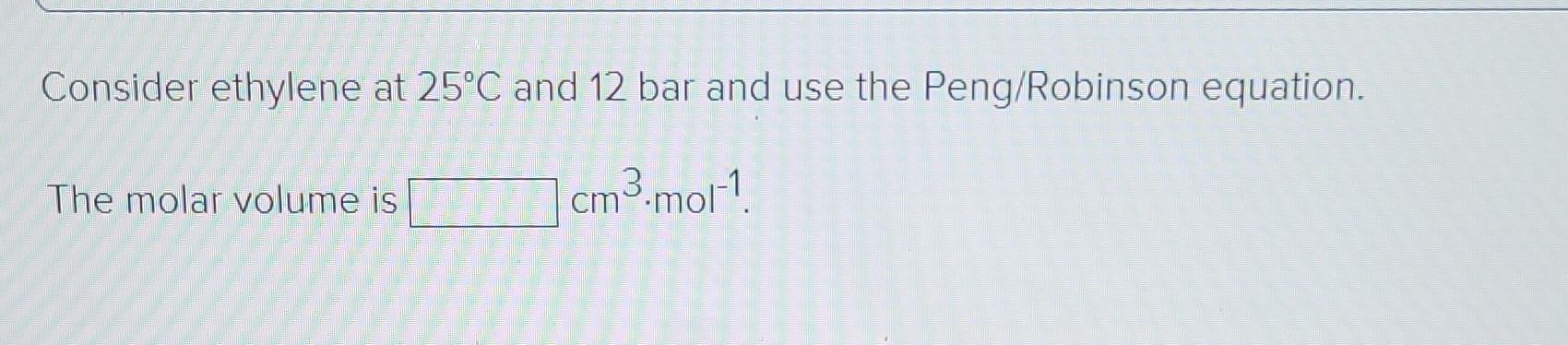
Required intormation The characteristic properties of ethylene are given in the following table: Molar Ve mass IK Po/bar Zo cm3.mol-! IK Ethylene 28.054 0.087 282.3 50.40 0.281 131. 169.4 The values of the ideal gas constant (R) are given below. Values of the Universal Gas Constant R = -1 8.314 J-mol-'.K-1 = 8.314 m.Pa.mol-1.K-1 83.14 cm3.bar-mol-'.K-1 8314 cmd.kPa.mol-'K-1 82.06 cm.(atm).mol-'K-! 62,356 cm (torr)-mol-'K- 1.987 (cal)-mol K-1 = 1.986 (Btu) (lb mole)- (R)- 0.7302 (ft)' (atm) (lb mol)- (R)-1 = 10.73 (ft) (psia) (lb mol)- (R)-! 1545 (ft) (lbe) (lb mol)--(R)-1 1 = II | -1 Calculate Zand V for the given condition. Consider ethylene at 25C and 18 bar and use the truncated virial equation with a value of B from the generalized Pitzer correlation. The molar volume is 1336.04 8 cm cm3.mol-1 The value of Z is 1.014 * Consider ethylene at 25C and 12 bar and use the Redlich/Kwong equation. The molar volume is 1 cm3 mot! Consider ethylene at 25C and 12 bar and use the Peng/Robinson equation. The molar volume is cm3.mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


