Answered step by step
Verified Expert Solution
Question
1 Approved Answer
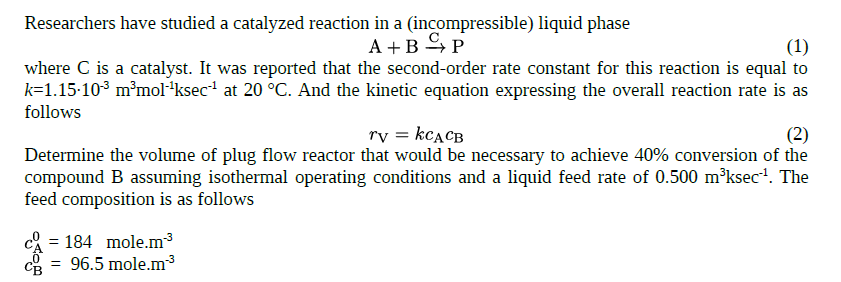
Researchers have studied a catalyzed reaction in a ( incompressible ) liquid phase A + B ( C ) P where C is a catalyst.
Researchers have studied a catalyzed reaction in a incompressible liquid phase
where is a catalyst. It was reported that the secondorder rate constant for this reaction is equal to
at And the kinetic equation expressing the overall reaction rate is as
follows
Determine the volume of plug flow reactor that would be necessary to achieve conversion of the
compound assuming isothermal operating conditions and a liquid feed rate of The
feed composition is as follows
mole.
mole. Please provide the complete solution to the problem specified in the attached document. If a calculation Excel is necessary, please attach these file
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


