Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Researchers indicated that the main reactions in the catalytic hydrogenation of cottonseed oil are: where A is linoleic acid, B is cis-oleic acid, C is
Researchers indicated that the main reactions in the catalytic hydrogenation of cottonseed oil are:
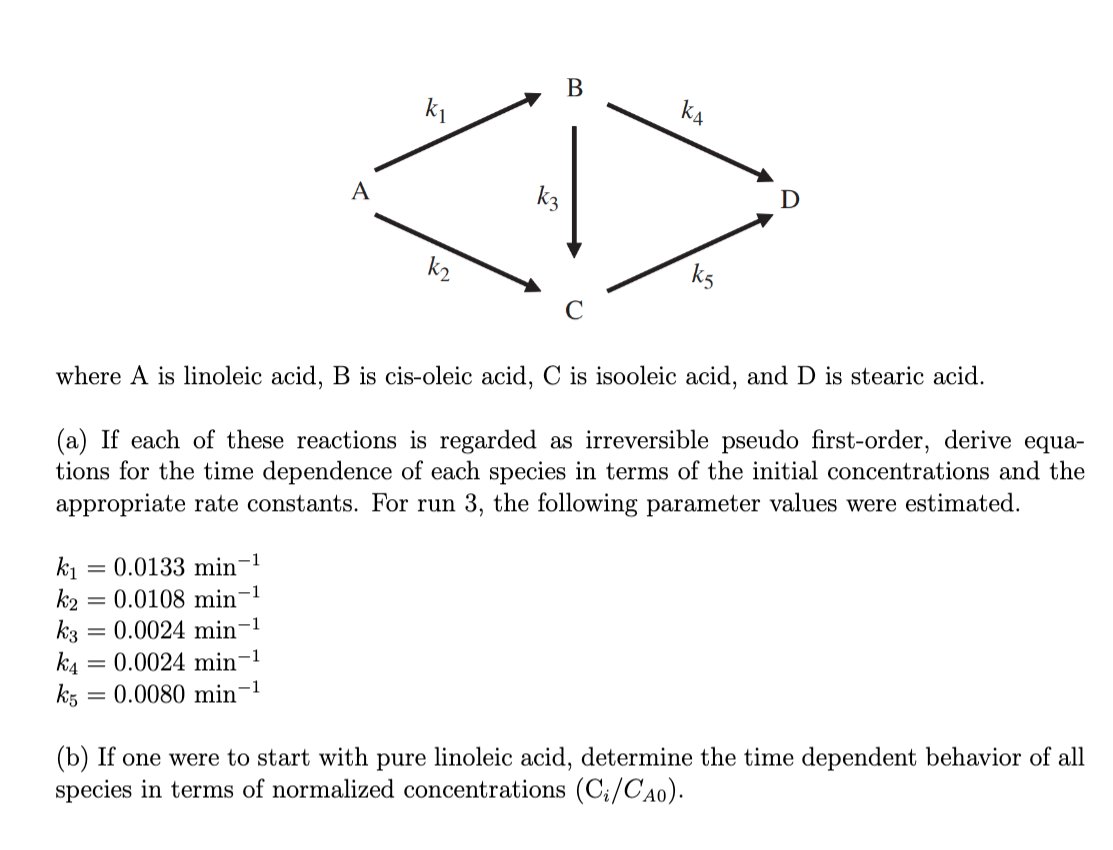 where A is linoleic acid, B is cis-oleic acid, C is isooleic acid, and D is stearic acid. (a) If each of these reactions is regarded as irreversible pseudo first-order, derive equations for the time dependence of each species in terms of the initial concentrations and the appropriate rate constants. For run 3 , the following parameter values were estimated. k1=0.0133min1k2=0.0108min1k3=0.0024min1k4=0.0024min1k5=0.0080min1 (b) If one were to start with pure linoleic acid, determine the time dependent behavior of all species in terms of normalized concentrations (Ci/CA0)
where A is linoleic acid, B is cis-oleic acid, C is isooleic acid, and D is stearic acid. (a) If each of these reactions is regarded as irreversible pseudo first-order, derive equations for the time dependence of each species in terms of the initial concentrations and the appropriate rate constants. For run 3 , the following parameter values were estimated. k1=0.0133min1k2=0.0108min1k3=0.0024min1k4=0.0024min1k5=0.0080min1 (b) If one were to start with pure linoleic acid, determine the time dependent behavior of all species in terms of normalized concentrations (Ci/CA0) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


