Question
Question The anode is where oxidation takes place. In a galvanic cell, the anode is negative. What is the charge of the anode in
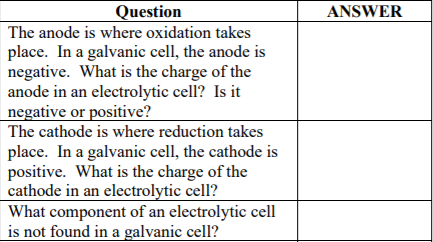
Question The anode is where oxidation takes place. In a galvanic cell, the anode is negative. What is the charge of the anode in an electrolytic cell? Is it negative or positive? The cathode is where reduction takes place. In a galvanic cell, the cathode is positive. What is the charge of the cathode in an electrolytic cell? What component of an electrolytic cell is not found in a galvanic cell? ANSWER
Step by Step Solution
3.49 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
In electrolytic cell we connect the positive terminal of battery in anode and negative terminal in ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus Early Transcendentals
Authors: James Stewart
8th edition
1285741552, 9781305482463 , 978-1285741550
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



