Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Respond to the following questions using the modified GRASP method, complete sentences and/or simple diagrams and illustrations whenever necessary and warranted. Pay attention to
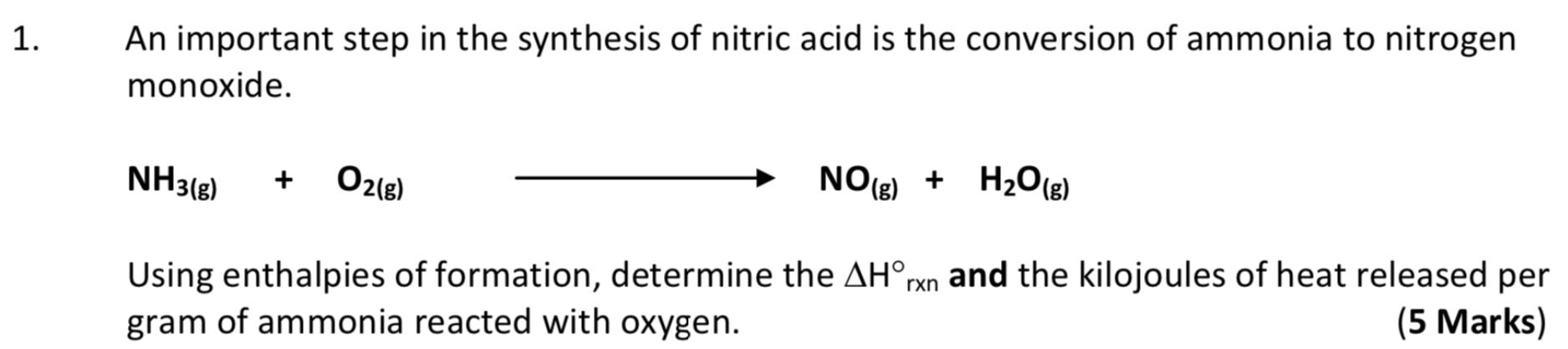
Respond to the following questions using the modified GRASP method, complete sentences and/or simple diagrams and illustrations whenever necessary and warranted. Pay attention to the mark value of each question. Remember to show all your work. 1. An important step in the synthesis of nitric acid is the conversion of ammonia to nitrogen monoxide. NH3(8) O2(g) NOg) + H20(e) + H2O(g) + Using enthalpies of formation, determine the AHrxn and the kilojoules of heat released per gram of ammonia reacted with oxygen. (5 Marks)
Step by Step Solution
★★★★★
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635e174de890c_181372.pdf
180 KBs PDF File
635e174de890c_181372.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started