Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Results 1. Using the analytical balance Mass of the stoppered flask (g) 49.3510 Mean mass (g) 44.35 Calculation: 49.3510+49.3473 +49.3461+ 49.3462 = 197.3906 =
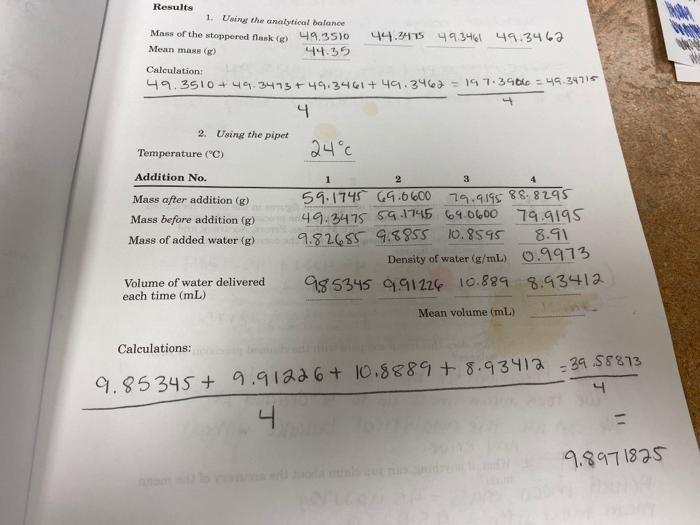


Results 1. Using the analytical balance Mass of the stoppered flask (g) 49.3510 Mean mass (g) 44.35 Calculation: 49.3510+49.3473 +49.3461+ 49.3462 = 197.3906 = 49.34715 2. Using the pipet Temperature (C) Addition No. Mass after addition (g) Mass before addition (g) Mass of added water (g) Volume of water delivered each time (mL) Calculations: 4 44.3475 49.3461 49.3467 24c 1 2 3 4 59.1745 69.0600 79.9195 88.8295 49.3475 59.1745 69.0600 79.9195 9.82685 9,8855 10.8595 8.91 0.9973 Density of water (g/mL) 985345 9.91226 10.889 8.93412 Mean volume (mL) 9.85345 + 9.91226 + 10.8889 +8.93412 39.58873 4 4 HOOP 9.8971825 WAX WE Questions 1. a. Calculate the standard deviation in the measured mass of the empty stoppered flask (see Appendix: Mistakes, Errors, Accuracy, and Precision). S mish Student name: 2. a. digit Course/Section: Date: Using the mean volume of water and the standard deviation, determine the number of significant figures that is allowed by the precision inherent in your pipet technique. Give the mean volume with the correct number of significant figures. 5.7x 103 0.0057 Standard deviation Rounding the standard deviation to single = 0.006. meaning the varability occurs in the thousand place (3rd decimal). Since the # of Significant b. Some claim that a precision of 10.01 mL can be achieved with a 10-ml pipet. However, students who are learning the technique of using a pipet may not achieve this precision. How do your results compare with the claim? If you have not been able to obtain this precision, try to pinpoint the deficiencies in your pipet technique.
Step by Step Solution
★★★★★
3.45 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


