Answered step by step
Verified Expert Solution
Question
1 Approved Answer
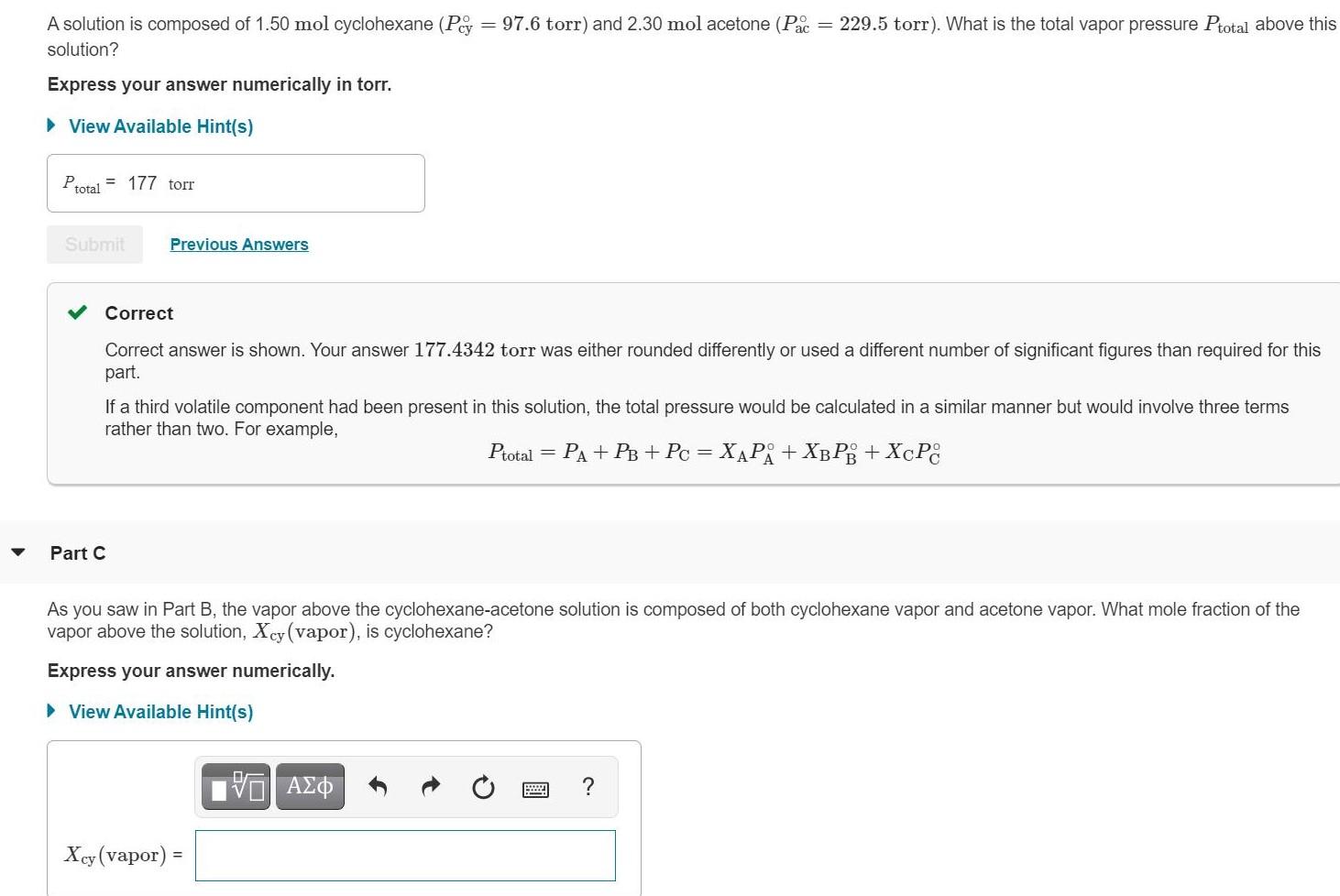
A solution is composed of 1.50 mol cyclohexane (Pey = 97.6 torr) and 2.30 mol acetone (Pac = 229.5 torr). What is the total
A solution is composed of 1.50 mol cyclohexane (Pey = 97.6 torr) and 2.30 mol acetone (Pac = 229.5 torr). What is the total vapor pressure Ptotal above this solution? Express your answer numerically in torr. View Available Hint(s) Ptotal = 177 torr Submit Previous Answers Correct Correct answer is shown. Your answer 177.4342 torr was either rounded differently or used a different number of significant figures than required for this part. If a third volatile component had been present in this solution, the total pressure would be calculated in a similar manner but would involve three terms rather than two. For example, Ptotal = + + 3D + + cC Part C As you saw in Part B, the vapor above the cyclohexane-acetone solution is composed of both cyclohexane vapor and acetone vapor. What mole fraction of the vapor above the solution, Xcy (vapor), is cyclohexane? Express your answer numerically. > View Available Hint(s) ? Xey (vapor): !!
Step by Step Solution
★★★★★
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started