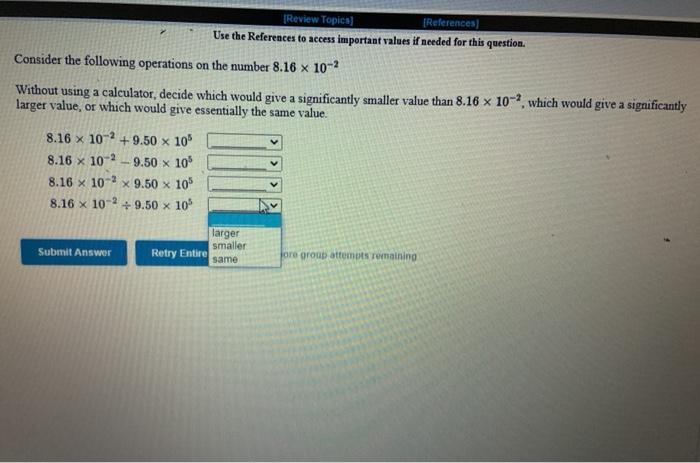
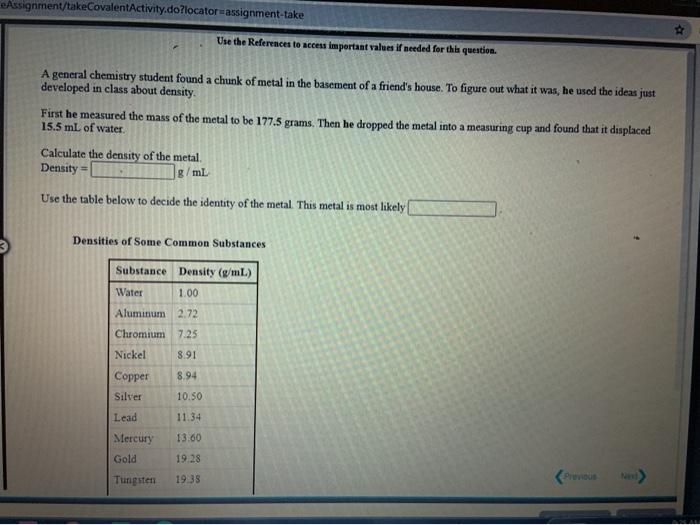
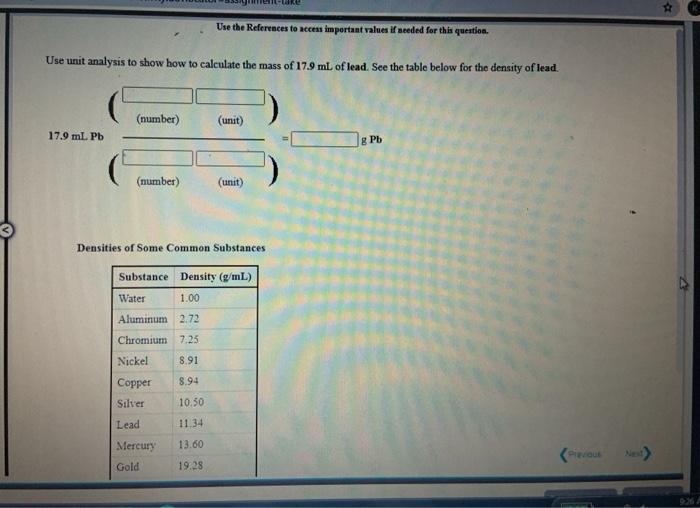
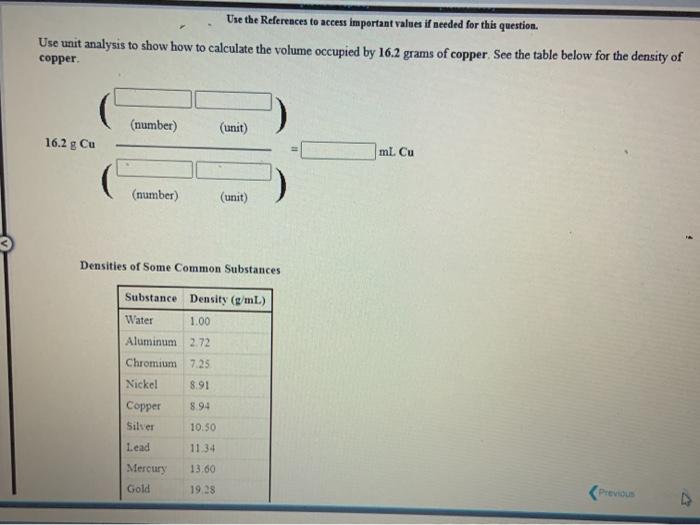
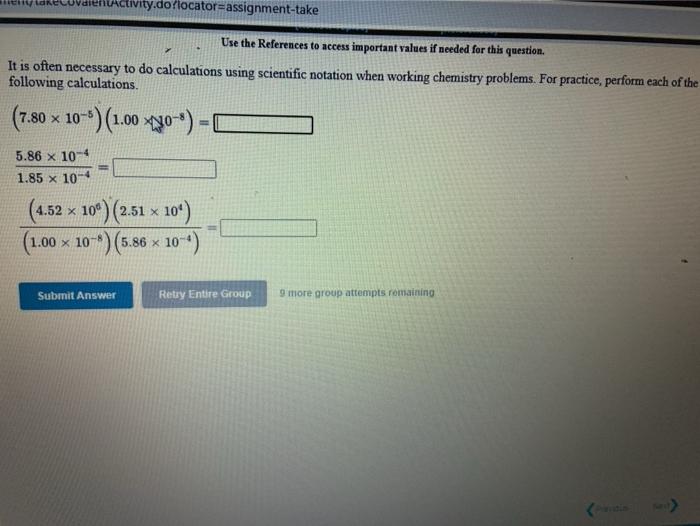
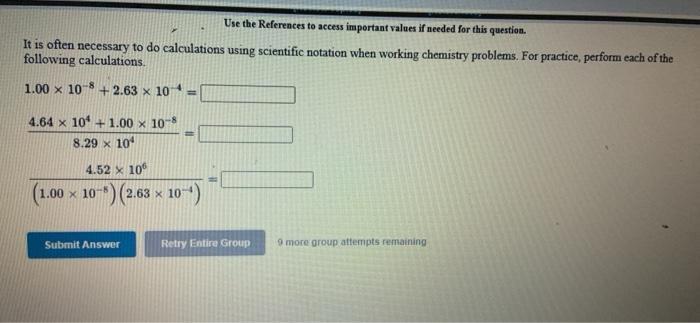
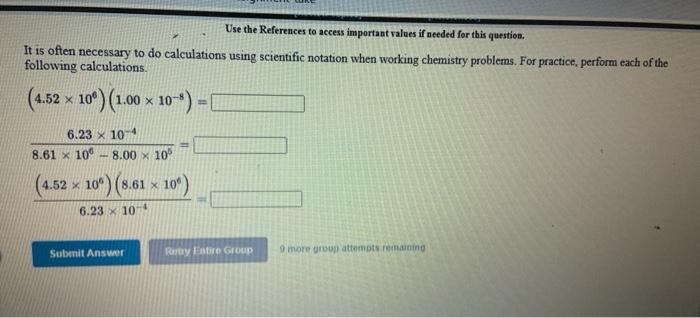
[Review Topics] [References) Use the References to access important values if needed for this question. Consider the following operations on the number 8.16 x 10-2 Without using a calculator, decide which would give a significantly smaller value than 8.16 x 10-2, which would give a significantly larger value, or which would give essentially the same value. 8.16 x 10-2 +9.50 x 10 8.16 x 10-29.50 x 10 8.16 x 10-? x 9.50 x 10% 8.16 x 10 -2 +9.50 x 10% larger Submit Answer smaller Retry Entire same jore group attempts remaining Assignment/takeCovalentActivity.do?locator assignment-take Use the References to access important values if needed for this question A general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, he used the ideas just developed in class about density First he measured the mass of the metal to be 177.5 grams. Then he dropped the metal into a measuring cup and found that it displaced 15.5 mL of water. Calculate the density of the metal. Density g/ml Use the table below to decide the identity of the metal This metal is most likely Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 Nickel 8.91 8.94 Copper Silver 10.50 11.34 Lead Mercury 13.60 Gold 19.28 Tungsten 19 38 * Use the References to access important values if needed for this question. Use unit analysis to show how to calculate the mass of 17.9 ml of lead. See the table below for the density of lead. (number) (unit) 17.9 ml Pb g Pb (number) (unit) Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 Nickel 8.91 Copper 8.94 Silver 10.50 11 34 Lead 13.60 Mercury Gold 19.28 Use the References to access important values if needed for this question. Use unit analysis to show how to calculate the volume occupied by 16.2 grams of copper See the table below for the density of copper. (number) (unit) 16.2 g Cu ml Cu (number) (unit) Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 Nickel 8.91 8.94 Copper Silver 10.50 Lead 11.34 13.60 Mercury Gold 19.28 LON Activity.do/locator=assignment-take Use the References to access important values if needed for this question. It is often necessary to do calculations using scientific notation when working chemistry problems. For practice, perform each of the following calculations. (7.80 x 10-*)(1.00 xxo-*) = 5.86 x 10-4 1.85 x 10-4 (4.52 * 10)(2.51 x 104) (1.00 x 10-) (5.86 x Submit Answer Retry Entire Group 9 more group attempts romaining Use the References to access important values if needed for this question. It is often necessary to do calculations using scientific notation when working chemistry problems. For practice, perform each of the following calculations 1.00 x 10-8 +2.63 x 10+ = 4.64 x 10 +1.00 x 10-8 8.29 X 10' 4.52 x 10 (1.00 x 10-6) (2.63 x 10) Submit Answer Retry Entire Group 9 more group attempts remaining Use the References to access important values if needed for this question. It is often necessary to do calculations using scientific notation when working chemistry problems. For practice, perform each of the following calculations. (4.52 x 10^)(1.00 x 10- ) - 6.23 x 10-4 8.61 x 10 8.00 x 10 (4.52 x 10") (8.61 x 10") 6.23 x 10-4 Submit Answer Roy Entire Group o more group attempts remaining