Answered step by step
Verified Expert Solution
Question
1 Approved Answer
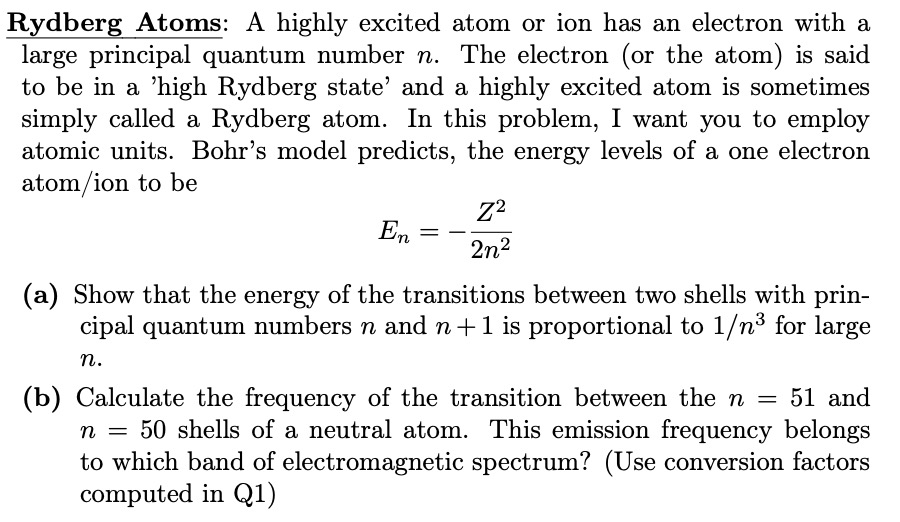
Rydberg Atoms: A highly excited atom or ion has an electron with a large principal quantum number n . The electron ( or the atom
Rydberg Atoms: A highly excited atom or ion has an electron with a
large principal quantum number The electron or the atom is said
to be in a 'high Rydberg state' and a highly excited atom is sometimes
simply called a Rydberg atom. In this problem, I want you to employ
atomic units. Bohr's model predicts, the energy levels of a one electron
atomion to be
a Show that the energy of the transitions between two shells with prin
cipal quantum numbers and is proportional to for large
b Calculate the frequency of the transition between the and
shells of a neutral atom. This emission frequency belongs
to which band of electromagnetic spectrum? Use conversion factors
computed in Q
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


