Answered step by step
Verified Expert Solution
Question
1 Approved Answer
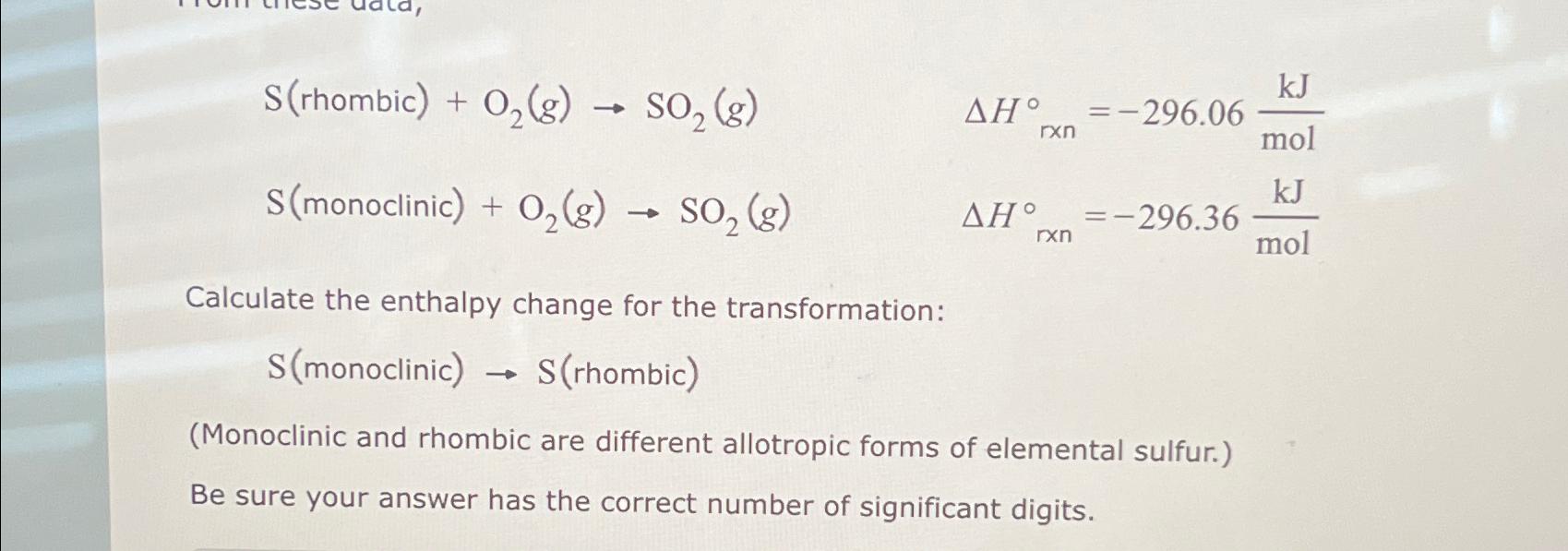
S( rhombic )+O_(2)(g)->SO_(2)(g),Delta H_(rtimes n)deg =-296.06(kJ)/(mol) S( monoclinic )+O_(2)(g)->SO_(2)(g),Delta H_(rtimes n)deg =-296.36(kJ)/(mol) Calculate the enthalpy change for the transformation: S (monoclinic) -> S(rhombic)
S( rhombic )+O_(2)(g)->SO_(2)(g),\\\\Delta H_(r\\\\times n)\\\\deg =-296.06(kJ)/(mol)\ S( monoclinic )+O_(2)(g)->SO_(2)(g),\\\\Delta H_(r\\\\times n)\\\\deg =-296.36(kJ)/(mol)\ Calculate the enthalpy change for the transformation:\
S (monoclinic) -> S(rhombic) \ (Monoclinic and rhombic are different allotropic forms of elemental sulfur.)\ Be sure your answer has the correct number of significant digits.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


