Answered step by step
Verified Expert Solution
Question
1 Approved Answer
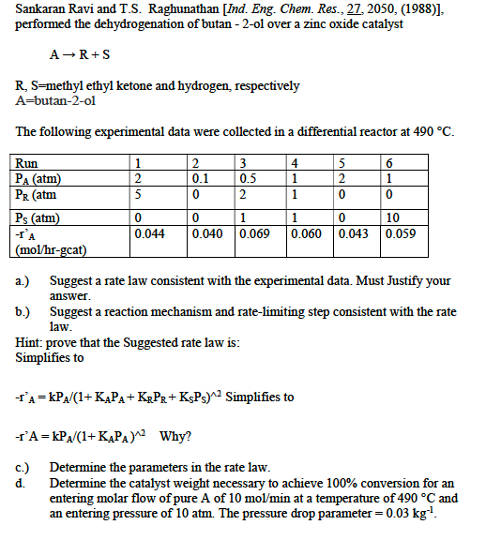
Sankaran Ravi and T . S . Raghunathan [ Ind . Eng. Chem. Res., 2 7 , 2 0 5 0 , ( 1 9
Sankaran Ravi and TS Raghunathan Ind Eng. Chem. Res.,
performed the dehydrogenation of butan ol over a zinc oxide catalyst
methyl ethyl ketone and hydrogen, respectively
butanol
The following experimental data were collected in a differential reactor at
a Suggest a rate law consistent with the experimental data. Must Justify your
answer.
b Suggest a reaction mechanism and ratelimiting step consistent with the rate
law.
Hint: prove that the Suggested rate law is:
Simplifies to
Simplifies to
Why?
c Determine the parameters in the rate law.
d Determine the catalyst weight necessary to achieve conversion for an
entering molar flow of pure of at a temperature of and
an entering pressure of atm. The pressure drop parameter
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


