Question
Scientists did experimental work on determination of equilibrium constants Keq for the decomposition of ammonium chloride: NH,Cl = NH3 + HCL Researchers, decided to
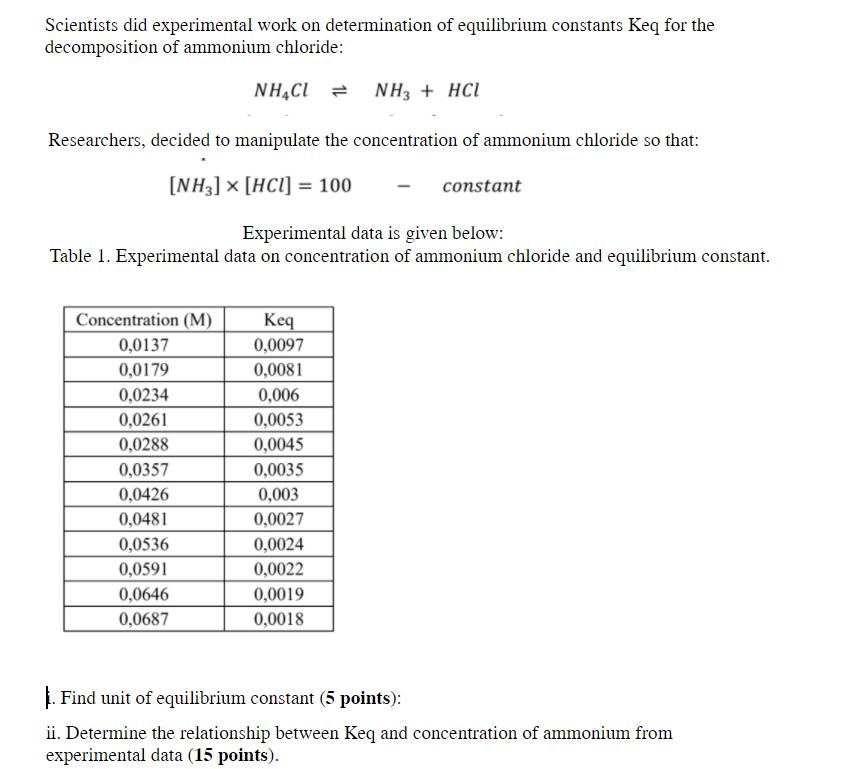
Scientists did experimental work on determination of equilibrium constants Keq for the decomposition of ammonium chloride: NH,Cl = NH3 + HCL Researchers, decided to manipulate the concentration of ammonium chloride so that: [NH3] x [HCl] = 100 constant Experimental data is given below: Table 1. Experimental data on concentration of ammonium chloride and equilibrium constant. Concentration (M) Keq 0,0097 0,0137 0,0179 0,0081 0,0234 0,006 0,0261 0,0053 0,0288 0,0045 0,0357 0,0035 0,0426 0,003 0,0481 0,0027 0,0536 0,0024 0,0591 0,0022 0,0646 0,0019 0,0687 0,0018 |. Find unit of equilibrium constant (5 points): ii. Determine the relationship between Keq and concentration of ammonium from experimental data (15 points).
Step by Step Solution
3.53 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Probability And Statistics For Engineering And The Sciences
Authors: Jay L. Devore
9th Edition
1305251806, 978-1305251809
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



