Answered step by step
Verified Expert Solution
Question
1 Approved Answer
second picture is a continuation of the first. use matlab code. Consider a nonisothermal batch reactor which is operated adiabatically. The reactor contains a liquid
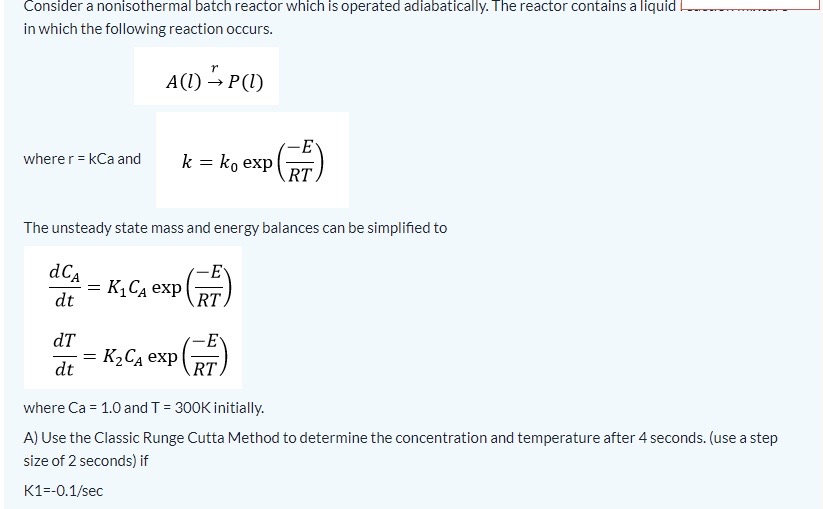
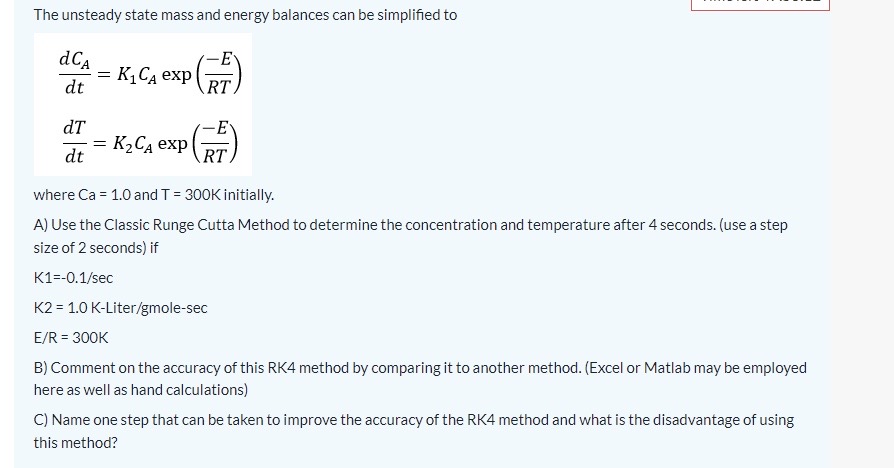
second picture is a continuation of the first. use matlab code.
Consider a nonisothermal batch reactor which is operated adiabatically. The reactor contains a liquid in which the following reaction occurs. A(l)rP(l) where r=kCa and k=k0exp(RTE) The unsteady state mass and energy balances can be simplified to dtdCA=K1CAexp(RTE)dtdT=K2CAexp(RTE) where Ca=1.0 and T=300K initially. A) Use the Classic Runge Cutta Method to determine the concentration and temperature after 4 seconds. (use a step size of 2 seconds) if K1=0.1/sec The unsteady state mass and energy balances can be simplified to dtdCA=K1CAexp(RTE)dtdT=K2CAexp(RTE) where Ca=1.0 and T=300K initially. A) Use the Classic Runge Cutta Method to determine the concentration and temperature after 4 seconds. (use a step size of 2 seconds) if K1=0.1/secK2=1.0KLiter/gmolesecE/R=300K B) Comment on the accuracy of this RK4 method by comparing it to another method. (Excel or Matlab may be employed here as well as hand calculations) C) Name one step that can be taken to improve the accuracy of the RK4 method and what is the disadvantage of using this method? Consider a nonisothermal batch reactor which is operated adiabatically. The reactor contains a liquid in which the following reaction occurs. A(l)rP(l) where r=kCa and k=k0exp(RTE) The unsteady state mass and energy balances can be simplified to dtdCA=K1CAexp(RTE)dtdT=K2CAexp(RTE) where Ca=1.0 and T=300K initially. A) Use the Classic Runge Cutta Method to determine the concentration and temperature after 4 seconds. (use a step size of 2 seconds) if K1=0.1/sec The unsteady state mass and energy balances can be simplified to dtdCA=K1CAexp(RTE)dtdT=K2CAexp(RTE) where Ca=1.0 and T=300K initially. A) Use the Classic Runge Cutta Method to determine the concentration and temperature after 4 seconds. (use a step size of 2 seconds) if K1=0.1/secK2=1.0KLiter/gmolesecE/R=300K B) Comment on the accuracy of this RK4 method by comparing it to another method. (Excel or Matlab may be employed here as well as hand calculations) C) Name one step that can be taken to improve the accuracy of the RK4 method and what is the disadvantage of using this methodStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


